|
There are currently five different types of reaction that may be simulated in HYSYS and a number of reactor types that they may be used with (and one special reactor that does not require any equations). Reactions may also be used in Columns and Separators (though there are some limitations on the phases that may be used by the reactions in those cases). The five reaction types are as follows: ConversionThis reaction type does not require any thermodynamic knowledge. You must input the stoichiometry and the conversion of the basis reactant. The specified conversion cannot exceed 100%. The reaction will proceed until either the specified conversion has been reached or a limiting reagent has been exhausted. EquilibriumEquilibrium reactions require that you know some sort of relation between the reaction's equilibrium constant, Keq, and temperature. You may specify Keq in a number of ways: Kinetic
Kinetic (Rev Eqm)
Langmuir-HinshelwoodThis is the most complicated of all the reaction forms and is therefore the one that is not even mentioned in any of the manuals (either on-line or off). Therefore it falls to me to explain it. I go through all of this in detail in the Plug Flow Example, so if you decide you do need to use this form, I recommend you work through that. General Information on ReactionsIf you are looking for a step by step instruction on every stage of the creation of a reaction and the use of reaction sets, you won't find it here. You will find explanations of that stuff in any of my reactor examples (Plug Flow Example, Gibbs and Equilibrium Reactors, Conversion Reactor), or you may look in Chapter 11 of Reference Volume 1 (does an excellent job with this kind of stuff), or work through the Chemicals Tutorial in the Tutorials Book. What I am going to list here are the little pearls of wisdom I picked up while working through the reactions myself and that may or may not be mentioned in the manuals.
|
|
Appearance in PFD / Object Palette Button |
Description |
|---|---|

 |
The Plug Flow Reactor can be used with Kinetics, Kinetics (Rev. Eqm.), or Langmuir-Hinshelwood reactions (any number and combination of the three types can be used in the reaction set). An excellent description of each of the PFR's inputs can be found in Section 13.10 of RV 2. You can also get a good idea of the way to go about setting up a PFR in your simulation by working through my Plug Flow Example. Quirks of the PFR: HYSYS "integrates"
over the length of the reactor by dividing it into
a number of sub-volumes (like a series of CSTRS).
The default is 20 sub-volumes. The most noticeble
effect of this to the user is in the reactor
profiles. The various characteristics are given as
values vs reactor length. The lengths listed are
the midpoimts of the subvolumes. For example, a 10
m length reactor with 20 subdivisions would give
profiles starting at .25 m and incrementing by .5 m
(the size of a subdivision), finishing with 9.75 m.
Not realizing this at first I was irritated that
the profiles were not showing me the entering and
exiting values (0 and 10 m). Not to worry, the .25
and 9.75 m values are, in fact, the same as 0
and 10 m. Everything within a subvolume is the
same (like a CSTR). |

 |
The CSTR can be used with Kinetics, Kinetics (Rev. Eqm.), or Langmuir-Hinshelwood reactions (any number and combination of the three types can be used in the reaction set). An excellent description of each of the CSTR's inputs can be found in Section 13.13.3 of RV 2. You can also get a good idea of the way to go about setting up a CSTR in your simulation by working through the Chemicals Tutorial in the Tutorials Book. In addition you might want to take a look at my Case Studies Example, where I build on the tutorial by adding a case study. I use the Spreadsheet feature to access the actual conversion % of the CSTR in the tutorial. Quirks of the CSTR: CSTR is primarily for liquid reactions, of course, but HYSYS will conduct the gaseous reactions as well. The less the "liquid" volume, the more of the total volume available for the vapour phase reactions (i.e. HYSYS uses the total volume minus the volume you set for the liquid to calculate the volume of the gas, whether or not any liquid is actually present in the stream). |
 opens opens
then, |
The Gibbs
Reactor (like the one in Aspen) is unique among
the reactors in that you are not required to enter
a reaction set for it to work. The Gibbs reactor
works by finding the equilibrium state with the
lowest Gibbs Free Energy. It appears to be akin to
finding all the possible equilibrium reactions and
allowing them all to equilibrate. It's nice because
you do not need to know anything about the
individual equilibrium constants. On the
Composition page you can set the production
of components or set any of them to be inert. Quirks of the Gibbs
Reactor: There is something
very important to note when attaching
equilibrium reactions to the Gibbs reactor. The
Gibbs reactor takes only the stoichiometry of the
attached reactions and applies its own free energy
minimization technique to it. Only
components listed as reacting in the reaction set
undergo any reaction. Note that HYSYS will not
allow you to attach a reaction set which would
include all of the possible independent reactions
as that would simply duplicate the effect of
setting the reactor to full Gibbs reactions. The
part of this that is important to you in the design
classes is that the results of the Gibbs
calculations come extremely close to the
values obtained in the equilibrium reactor using
correct data, while not making use of any
data on Keq. Thus if you need to
simulate a reactor in which you want certain
reactions equilibrated, but not others (for
instance, because a certain catalyst is employed
allowing those particular reactions to equilibrate
quickly, but not aiding any other reaction) and yet
have no or untrustworthy data on the equilibrium
constants, you are better off using the Gibbs
reactor set on "Specify Equilibrium Reaction" than
using the Equilibrium Reactor. One last note, there appears to be a minor bug in HYSYS, in that, when operating the Gibbs Reactor in Equilibrium Reactor mode, a button appears that would show you the % conversion, reaction extent, etc. Unfortunately, even when the Gibbs Reactor had completed its calculations, the matrix remained blank. If you would like to experiment with the similarities and differences between the Gibbs reactor and the Equilibrium Reactor yourself, see the example for a good way to go about it. |
 opens opens
then, |
The Equilibrium Reactor uses reaction sets with only, surprise, equilibrium reactions in it. You can read more about it in Section 13.13.4 of RV 2. You can also see the example in which I compare it to a Gibbs Reactor. In general, I recommend making use of the Gibbs Reactor over the Equilibrium Reactor. |
 opens opens
then, |
The Conversion Reactor deals with, yep, you guessed it, conversion reactions. You use it when you know how much of the reactants will be converted into products. As mentioned in the section on conversion reactions, it can handle multiple reactions which may be ranked to occur simultaneously or sequentially. Reactions with the same ranking are simultaneous and the total conversion of the same reactant can not exceed 100% (all subject to limiting reagents, of course). The product of one reaction can be the reactant of another reaction. Quirks of the Conversion
Reactor: Though the specified
conversion cannot exceed 100%, the actual
conversion can. This is because the actual
conversion is the percentage conversion over the
original amount of base component present.
However, if that base component is the
product of a lower ranked (meaning reacts
first) reaction, there may be more available than
was originally there. This allows the actual
conversion to exceed the specified conversion (it's
still behaving correctly, so don't panic). The
conversion could thus be much greater than 100%.
You can see this in my conversion
example. If none of the base component was
initially present, the actual conversion field will
remain blank. Conversely, if the base component of
a reaction is a reactant in an earlier
occuring reaction, or if there are limiting
reagents, the actual conversion will be less than
the specified conversion. |
Not Accessible from the Object Palette. Can only be reached via the Unit Ops View (obtainable by pressing <F12>, "Add an Operation" under the Flowsheet Menu, or from the Unit Ops Page of the Workbook. |
The General Reactor is like a combination of the CSTR and the Equilibrium Reactor. If you put in all kinetic style reactions, it acts like a CSTR. If you put in all equilibrium reactions, it acts like an Equilibrium Reactor. The General Reactor, unlike any other type of reactor available to you, will also allow you to mix equation types. You can combine kinetic and equilibrium reactions into one reaction set (you still cannot combine conversion reactions with any other type, though you can attach a conversion reaction set to the general reactor as well). That set will then be a mixed type and can be attached to the general reactor. Unfortunately, verifying the accuracy and method is somewhat involved (though not necessarily difficult). I leave it to you to investigate this. Rather than create an example for you. This time I simply added the General Reactor to my file comparing all the other reactors. That way, you may use all the reactions and reaction sets that were created for the other examples as a way of exploring the results that the General Reactor gives you. The name of the file is AllReactors.hsc and it is located under \\Hartsook\Hysys\SAMP403. Quirks of the General
Reactor: A strange problem, somehow
tied into the number of product streams attached,
results if you try to attach an equilibrium set
immediately after a conversion set. It fails to
find a solution even if it had done so before. If
something like this happens to you click off then
back on again the Act as a Separator when cannot
solve button and it should solve. |
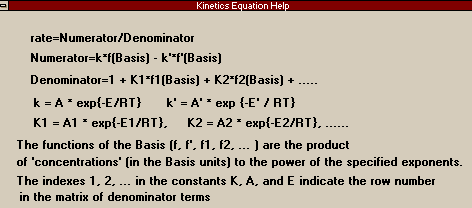
 All
three of the remaining reaction types can be considered
kinetic, in that they deal with an expression for the
rate of the reaction. Differentiating between the three
becomes simply a matter of formulation. In this first and
simplest form, the rate equation is the one to the left
(this picture is taken from the Parameters Page of
the Kinetics Reaction window). The first term on the
right hand side refers to the forward reaction, the
second term to the optional backward reaction. The k's
are the reaction constants for which you must enter on
the Parameters Page the activation energies, E and E',
and the pre-exponential factors, A and A' (which are
basically all of the constants lumped out front). The
basis functions are not just functions of the Base
Component (which you set on the Basis Page -- see
Chapter 11 of RV1 for an
explanation of the Base Component or anything else having
to do with reactions), but are the products of the
concentrations (or partial pressures, etc.) of any of the
reactants or products to whatever power (negative numbers
and decimals are fine). For example, it just so happens
that for the reaction
All
three of the remaining reaction types can be considered
kinetic, in that they deal with an expression for the
rate of the reaction. Differentiating between the three
becomes simply a matter of formulation. In this first and
simplest form, the rate equation is the one to the left
(this picture is taken from the Parameters Page of
the Kinetics Reaction window). The first term on the
right hand side refers to the forward reaction, the
second term to the optional backward reaction. The k's
are the reaction constants for which you must enter on
the Parameters Page the activation energies, E and E',
and the pre-exponential factors, A and A' (which are
basically all of the constants lumped out front). The
basis functions are not just functions of the Base
Component (which you set on the Basis Page -- see
Chapter 11 of RV1 for an
explanation of the Base Component or anything else having
to do with reactions), but are the products of the
concentrations (or partial pressures, etc.) of any of the
reactants or products to whatever power (negative numbers
and decimals are fine). For example, it just so happens
that for the reaction This
form of the rate equation is fairly similar to the
standard kinetic form. The difference is that instead of
getting information about the reverse rate constant, we
use the relation:
This
form of the rate equation is fairly similar to the
standard kinetic form. The difference is that instead of
getting information about the reverse rate constant, we
use the relation: