The problem can be solved in two steps: 1) use a mass balance on the system and the equilibrium expression for the reaction to find the exit temperature, 2) use the energy balance to determined the amount of heat that needs to be removed.
1) The steady-state macroscopic mass balance for each component can be expressed in Equation 23.1-3:
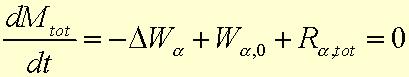
![]()
where
Wa is the molar flow rate for each component a
Ra is the molar rate of production of species a
The stoichiometric relationships can be utilized:
![]()
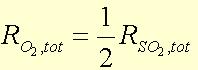
From all of this, the exit flow rates of each component can be determined. The equilibrium constant, Kp, is then calculated using the given equation:
The partial pressure of each component is equal to its mole fraction multiplied by the total pressure. However, in this problem the exit pressure is 1.00 atm, therefore mole fractions can be substituted for partial pressures. The mole fraction of each species is equal to its molar flow rate divided by the total molar flow rate.
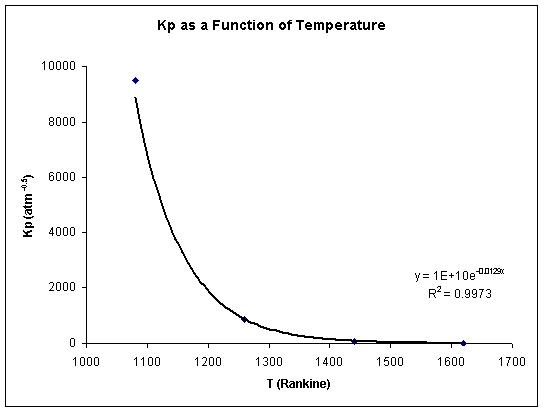
Once the equilibrium constant is determined, the exit temperature necessary to achieve this Kp value is found using a correlation of the data given on page 741 of Bird, Stewart, and Lightfoot.
2) The macroscopic statement of the law of conservation of energy is Equation 23.3-1:
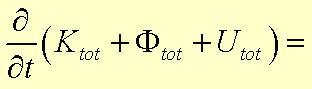 |
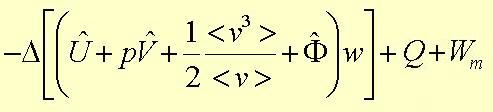 |
|---|
where
U is the internal energy
K is the kinetic energy
F is the potential energy
H is the enthalpy flow rate (H=U+pV)
w is the mass flow rate
Q is the heat flow rate
Wm is the rate of work done by moving surfaces
With the following assumptions, the energy balance can be simplified:
1.) We want the steady state solution. Therefore, the entire left side of the equation is zero.
2.) The changes in potential and kinetic energy are negligible relative to the change in enthalpy.
3.) No work is done by moving surfaces.
4.) Ideal gas behavior is assumed, which allows each constinuent's enthalpy to be:
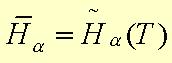
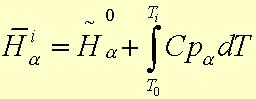
Cp is the heat capacity of species a over the range of temperatures
The energy balance, after all of the simplifications and assumptions, reduces to:
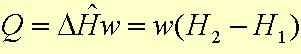
which can be solved to find Q, the amount of heat that must be removed from or added to the system in order to permit the specified operating conditions.
The following Maple calculations will show that in order to achieve a 95% conversion with an inlet temperature of 440 ºC (1283.67 R), 1.598e5 Btu/hr heat must be removed from the converter.


