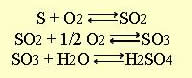- THE SO2 OXIDATION REACTION
- Exothermic ( -42000 Btu / lbmol of SO2)
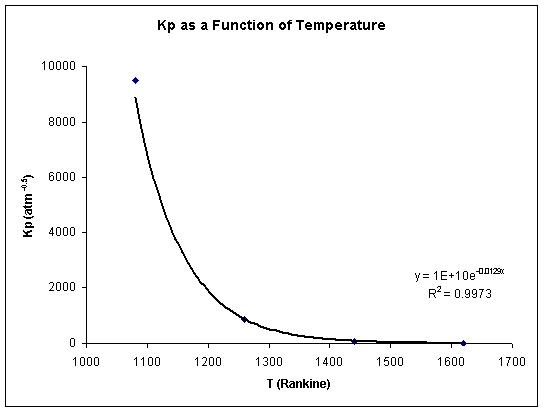
- Equilibrium conversion drops as temperature increases
- Multiple converter passes with interstage cooling are used to increase conversions, which approach 99.7% in industry reactors.
- Production of sulfuric acid
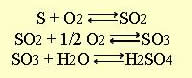
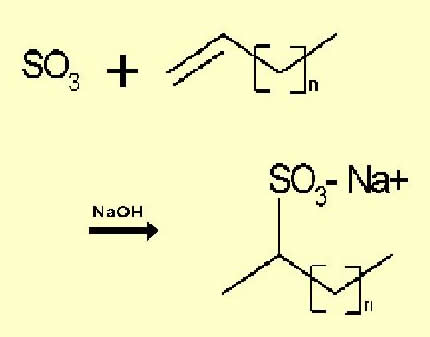
- Sulfonation of many types of organic compounds (to produce surfactants, soaps, and shampoo ingredients)
- IMPORTANT FACTS
- The burning of S to form SO2 is very exothermic; therefore the feed to the converter is cooled in order to increase conversion.
- The air used in the reactor must have an extremely low moisture content. If water is present, sulfuric acid and oleum (fuming sulfuric acid) will form in the converter and cause corrosion.
top
|