|
|
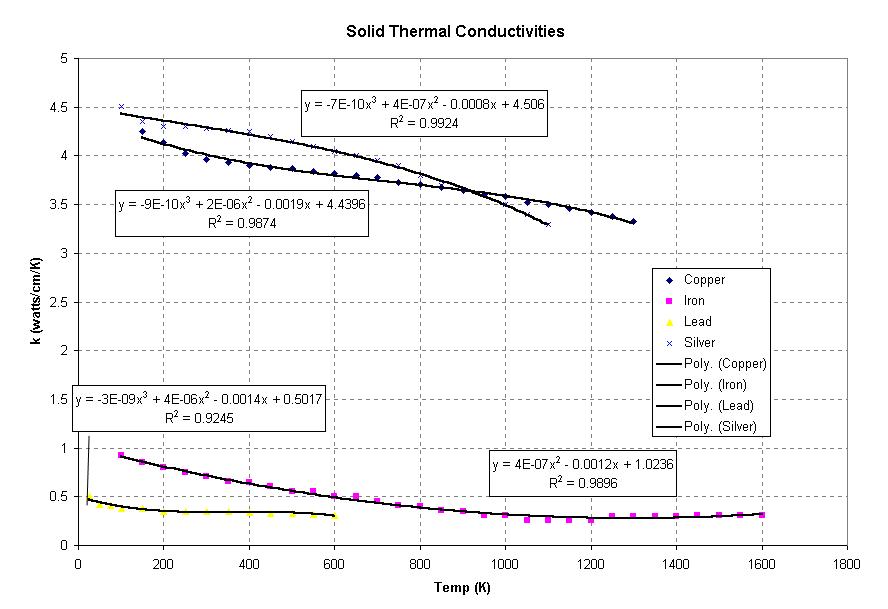
In order to calcuate the thermal conductivity of liquids, appoximations had to be made. Also, data were difficult to find. This problem intensifies for calculating the thermal conductivities of solids. Using the work of John Cliver and Catherine Hoang, we calculated these thermal conductivities using curve fitting to already tabulated thermal conductivities. (http://www.owlnet.rice.edu/~ceng402/proj03/choang/ceng402/ceng402.html) Here is an example of some of the data that they fitted curves to.
Third order curve fitting was applied to the ten solids listed below and put in a program called kscalc.
To verify that this approach gave accurate results, an example from the book was used with Aluminum as the solid. >> help kscalc.m calculates the thermal conductivity of solids >> kcalc(573.2,2,'s',6) ans = 231.8211 Answer from the book (pg. 271)
|
Home![]() Gases
Gases![]() Liquids
Liquids![]() Solids
Solids![]() Sources
Sources
![]() © 2003
Finley and Dunnavant
© 2003
Finley and Dunnavant ![]()
Template
Designed by JSB Web Templates