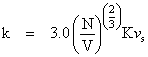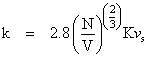|
|
The thermal conductivity for liquids can be determined using a modified version of Bridgman's equation. P.W. Bridgman assumed that liquid molecules are arranged in a cubic lattice and that energy is transferred from one lattice plane to the next at the speed at which sound travels through the fluid of interest. The following is Bridgman's equation:
In this equation, N (Avogadro's number) = 6.02e23 and V (molar volume) =
The modification of Bridgman's equation accounts better for polyatomic liquids, and simply involves decreasing the coefficient:
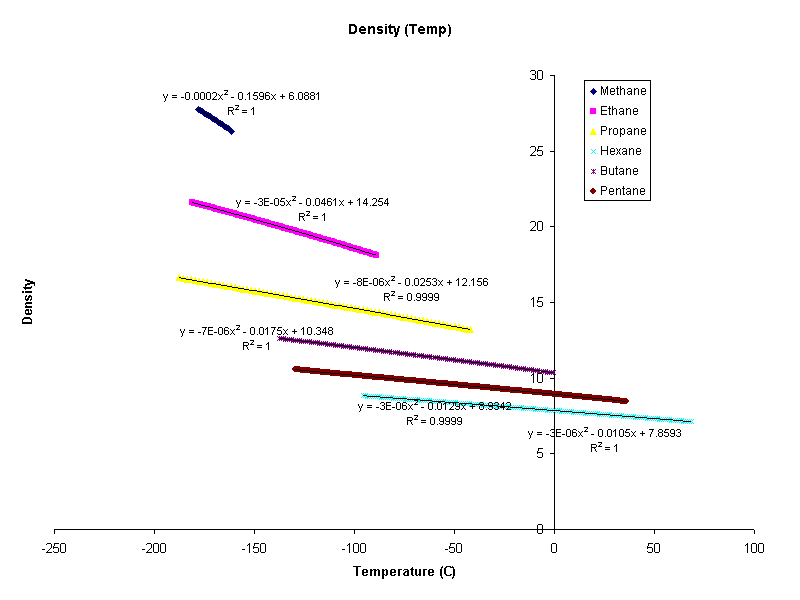
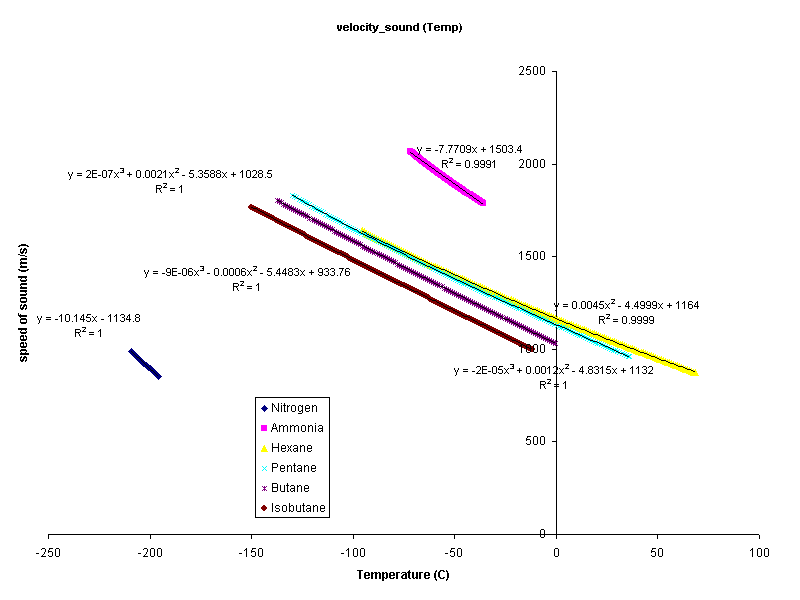
In order to find thermal conductivity certain approximations were made. Density and the velocity of sound are assumed to be functions of temperature alone, therefore they are independent of P. At low pressures this is a good assumption. A program called klcalc was created to calculate the thermal conductivities of liquids. Data were not available for many compounds. The following compounds have density and speed of sound data.
The data were fit to a curve as a function of temperature
All of the density figures showed very nearly linear behavior. Some for example, methane, were highly sensitive to temperature. It would be ridiculous to assume constant temperature in this scenario.
To check the accuracy of the kcalc program for liquids, examples from the book with methane and water were attempted. Water Example Here are your compounds' formulae and names: >> kcalc(300,1,'l') ans = 0.8453 Answer from book (pg. 270) The agreement here is rough.
Methane Example Here are your compounds' formulae and names: >> kcalc(103,2,'l') ans = 0.3564 Answer from http://webbook.nist.gov/ This result also gives only an approximation of what is happening in the system.
|
Home![]() Gases
Gases![]() Liquids
Liquids![]() Solids
Solids![]() Sources
Sources
![]() © 2003
Finley and Dunnavant
© 2003
Finley and Dunnavant ![]()
Template
Designed by JSB Web Templates