This section of the notes shows:
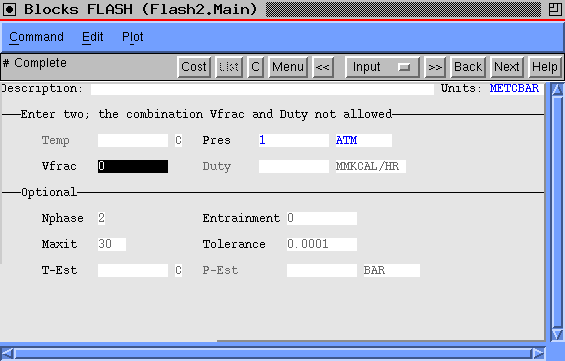
AspenPlus may be used to determine the bubble or dew point of a mixture with the FLASH2 module. The main form for the Flash2 module looks like:

If we specify the pressure and set the fraction vapor as zero as shown, we will produce the bubbple point for the mixture. In the case shown, we had a mixture of three compounds: acetone, methanol and isoproply alcohol. Executing the flash produced the following stream data:
Display ALLSTREAMS FEED LIQUID VAPOR
Units: From FLASH FLASH
Format: GEN_M To FLASH
Phas VAPOR LIQUID MISSING
Temperature [C] 70.0 64.5
Pressure [BAR] 1.013 1.013 1.013
Vapor Frac 1.000 0.000
Mole Flow [KMOL/HR] 100.000 100.000 0.000
Mass Flow [KG/HR] 4806.807 4806.807 0.000
Volume Flow [CUM/HR] 2815.753 6.427 0.000
Enthalpy [MMKCAL/H -5.210 -6.034
Mole Flow [KMOL/HR]
CH3OH 40.000 40.000
ACETONE 40.000 40.000
I-P 20.000 20.000
Mole Frac
CH3OH 0.400 0.400
ACETONE 0.400 0.400
I-P 0.200 0.200
That tells us that the bubble point is 64.5C, but it does not tell us the vapor composition. To find that we need to look at the FLASH Screen that can be found three clicks of the ">>" button after the Stream resurlts form.
Run ID: EX73IP Item: FLASH Screen: Flash2.VLE2
C-----------C-----------C----------C----------C----------C----------C-----------
Description: Units:
Component F X Y K
CH3OH .4000000 .4000000 .3948731 .9871790
ACETONE .4000000 .4000000 .5118005 1.279508
I-P .2000000 .2000000 .0933263 .4666271
The dew point may be found the same way, but this time we need to specify that Vfrac is 1. The stream flows are then:
Display ALLSTREAMS FEED LIQUID VAPOR
Units: From FLASH FLASH
Format: GEN_M To FLASH
Phas LIQUID MISSING VAPOR
Temperature [C] 70.0 75.5
Pressure [BAR] 1.013 1.013 1.013
Vapor Frac 0.000 1.000
Mole Flow [KMOL/HR] 100.000 0.000 100.000
Mass Flow [KG/HR] 5828.694 0.000 5828.694
Volume Flow [CUM/HR] 7.916 0.000 2860.654
Enthalpy [MMKCAL/H -6.657 -5.792
Mole Flow [KMOL/HR]
CH3OH 3.510 3.510
ACETONE 40.890 40.890
I-P 55.600 55.600
Mole Frac
CH3OH 0.035 0.035
ACETONE 0.409 0.409
I-P 0.556 0.556
The Dew point is 75.5C and the vapor composition is found again on the Flash2.VLE2 screen:
Component F X Y K CH3OH .0351000 .0235860 .0351000 1.488164 ACETONE .4089000 .2294523 .4089000 1.782033 I-P .5560000 .7469617 .5560000 .7443542
It is fortunate that this procedure is so simple and does not require the effort involved with a sensitiviety run described in the section of these notes on basic Aspen operations. It enabled us to construct the following lists comparing various thermodynamics packages with experimental data for the acetone, methanol, isopropyl alcohol system. The analysis of the is data started with the Matlab programs used to illustrate the fit of the data with Margules two suffix activity model. One program in that section of the notes compared the experimental data for the isobaric runs with the Margules approximation and with an ideal model. Another program compared the experimental data for the isothermal runs. We extended both of these to include several thermodymic packages in Aspen. Here are the results for four of the isobaric runs:
Comparison of Experimental Data for Acetone, Methanol, Isopropanol
Bubble Points and Vapor Compositions with various
Thermodynamic Packages
Part 1: Isobaric data at 1 atmospheres
Run Acetone MeOH IsoPOH Package
2 Liquid 0.0930 0.5230 0.3840
Bubble Pt C Vapor mol fractions
66.9589 0.2206 0.5768 0.2026 Margules
66.8400 0.2390 0.5810 0.1800 Expt
69.4174 0.1441 0.6292 0.2267 Ideal
69.9 0.1404 0.6334 0.2262 Van Laar No binary parms
67.35 0.2230 0.5780 0.1990 Van Laar With binary parms
65.9 0.2092 0.5801 0.2106 Wilson
64.3 0.1380 0.5922 0.2698 P-R
66.4 0.1565 0.6218 0.2218 PR-BM
65.9 0.2076 0.5813 0.2111 NRTL
66.1 0.2034 0.5868 0.2098 UNIFAC
66.0 0.2064 0.5814 0.2121 UNIQUAC
Run Acetone MeOH IsoPOH Package
9 Liquid 0.1324 0.2635 0.6041
Bubble Pt C Vapor mol fractions
69.4956 0.3182 0.3226 0.3592 Margules
69.2300 0.3218 0.3391 0.3391 Expt
72.8118 0.2280 0.3602 0.4118 Ideal
73.4 0.2223 0.3642 0.4135 Van Laar No binary parms
69.50 0.3162 0.3187 0.3651 Van Laar With binary parms
68.1 0.3117 0.3402 0.3481 Wilson
67.0 0.1932 0.3602 0.4466 P-R
70.0 0.2227 0.3880 0.3893 PR-BM
68.2 0.3104 0.3403 0.3493 NRTL
68.5 0.3056 0.3410 0.3534 UNIFAC
68.2 0.3090 0.3388 0.3523 UNIQUAC
Run Acetone MeOH IsoPOH Package
15 Liquid 0.3089 0.1895 0.5016
Bubble Pt C Vapor mol fractions
64.7333 0.5429 0.2051 0.2520 Margules
64.6500 0.5465 0.2040 0.2495 Expt
69.3407 0.4776 0.2273 0.2951 Ideal
70.1 0.4697 0.2317 0.2986 Van Laar No binary parms
64.56 0.5154 0.2231 0.2615 Van Laar With binary parms
63.8 0.5301 0.2160 0.2538 Wilson
64.8 0.4112 0.2500 0.3388 P-R
66.9 0.4556 0.2613 0.2831 PR-BM
63.9 0.5301 0.2153 0.2546 NRTL
64.0 0.5330 0.2127 0.2543 UNIFAC
64.0 0.5258 0.2152 0.2591 UNIQUAC
Run Acetone MeOH IsoPOH Package
20 Liquid 0.4089 0.0351 0.5560
Bubble Pt C Vapor mol fractions
64.9135 0.6651 0.0404 0.2945 Margules
64.7300 0.6596 0.0552 0.2852 Expt
69.3013 0.6314 0.0420 0.3266 Ideal
70.2 0.6241 0.0431 0.3328 Van Laar No binary parms
65.73 0.6358 0.0522 0.3121 Van Laar With binary parms
64.2 0.6618 0.0439 0.2943 Wilson
66.1 0.5536 0.0515 0.3949 Peng-Rob
68.3 0.6112 0.0540 0.3348 PR-BM
64.2 0.6616 0.0434 0.2949 NRTL
64.1 0.6664 0.0416 0.2920 UNIFAC
64.4 0.6547 0.0433 0.3020 UNIQUAC
The first three lines in the comparison were listed by the Matlab program buberg. The next six lines came from Aspen runs. The four run were chosen to cover a reasonable range of compositions and most of the cases where the the ideal and experimental data had the largest differences.
Here is a similar table of isothermal runs:
Comparison of Experimental Data for Acetone, Methanol, Isopropanol
Bubble Points and Vapor Compositions with various
Thermodynamic Packages
Part 2: Isothermal data at 55C
Run Acetone MeOH IsoPOH Package
3 Liquid 0.5372 0.3447 0.1181
Bub P: kPa Vapor mol fractions
93.0284 0.6459 0.3091 0.0449 Margules
92.7790 0.6480 0.3048 0.0472 Expt.
79.7977 0.6578 0.2962 0.0460 Ideal
78.2 0.6532 0.3007 0.0461 Van Laar No binary parms
92.93 0.6312 0.3216 0.0472 Van Laar With binary parms
93.2 0.6359 0.3116 0.0526 Wilson
89.5 0.5964 0.3430 0.0606 Peng-Rob
89.4 0.6159 0.3393 0.0448 PR-BM
93.1 0.6360 0.3117 0.0522 NRTL
93.1 0.6372 0.3107 0.0521 UNIFAC
93.0 0.6350 0.3125 0.0525 UNIQUAC
Run Acetone MeOH IsoPOH Package
13 Liquid 0.3453 0.6239 0.0308
Bub P: kPa Vapor mol fractions
91.9504 0.4855 0.5037 0.0107 Margules
91.3498 0.4856 0.5027 0.0117 Expt.
77.4747 0.4355 0.5521 0.0124 Ideal
76.3 0.4301 0.5575 0.0123 Van Laar No binary parms
91.62 0.4836 0.5053 0.0111 Van Laar With binary parms
91.5 0.4784 0.5081 0.0136 Wilson
89.6 0.4394 0.5437 0.0169 P-R
90.1 0.4545 0.5334 0.0121 PR-BM
91.5 0.4786 0.5080 0.0134 NRTL
91.4 0.4764 0.5102 0.0133 UNIFAC
91.5 0.4784 0.5081 0.0135 UNIQUAC
Run Acetone MeOH IsoPOH Package
16 Liquid 0.0633 0.5681 0.3686
Bub P: kPa Vapor mol fractions
61.0619 0.1738 0.6399 0.1863 Margules
61.9749 0.1817 0.6424 0.1759 Expt.
56.5937 0.1093 0.6882 0.2025 Ideal
56.0 0.1074 0.6919 0.2007 Van Laar No binary parms
60.70 0.1812 0.6382 0.1806 Van Laar With binary parms
63.9 0.1661 0.6386 0.1953 Wilson
69.3 0.1055 0.6314 0.2631 P-R
63.4 0.1246 0.6663 0.2091 PR-BM
63.8 0.1648 0.6397 0.1955 NRTL
63.5 0.1607 0.6460 0.1933 UNIFAC
64.0 0.1667 0.6372 0.1961 UNIQUAC
Run Acetone MeOH IsoPOH Package
24 Liquid 0.3127 0.2807 0.4066
Bub P: kPa Vapor mol fractions
74.3246 0.5438 0.2791 0.1770 Margules
74.5819 0.5532 0.2780 0.1688 Expt.
62.4407 0.4894 0.3082 0.2024 Ideal
61.3 0.4851 0.3125 0.2024 Van Laar No binary parms
75.41 0.5228 0.2931 0.1841 Van Laar With binary parms
76.7 0.5274 0.2880 0.1846 Wilson
74.4 0.4177 0.3325 0.2498 P-R
70.1 0.4634 0.3412 0.1955 PR-BM
76.5 0.5275 0.2876 0.1848 NRTL
76.2 0.5301 0.2862 0.1837 UNIFAC
76.6 0.5258 0.2871 0.1871 UNIQUAC
Here is a table that summarizes the data shown in the previous lists:
|
|
Sum of absolute errors in Bubble Points for 4 isobaric runs in C |
Sum of absolute errors in Bubble Pressures for 4 isothermal runs in kPa |
Sum of absolute errors in acetone vapor mol fractions for 8 runs |
|
Margules 2 suffix with three parameters fit to 48 runs and 3 activities/run |
|
|
|
|
Ideal package on Matlab |
|
|
|
|
Van Laar with no binary data in Aspen |
|
|
|
|
Van Laar with binary parameters from fit of experimental isbaric data |
|
|
|
|
Wilson with built-in binary data |
|
|
|
|
Peng-Robinson in Aspen |
|
|
|
|
Peng-Robinson, Boston-Mathias in Aspen |
|
|
|
|
NRTL in Aspen |
|
|
|
|
UNIFAC in Aspen |
|
|
|
|
UNIQUAC in Aspen |
|
|
|