|
Course Syllabus
|
GLHT 201: Bioengineering & World Health
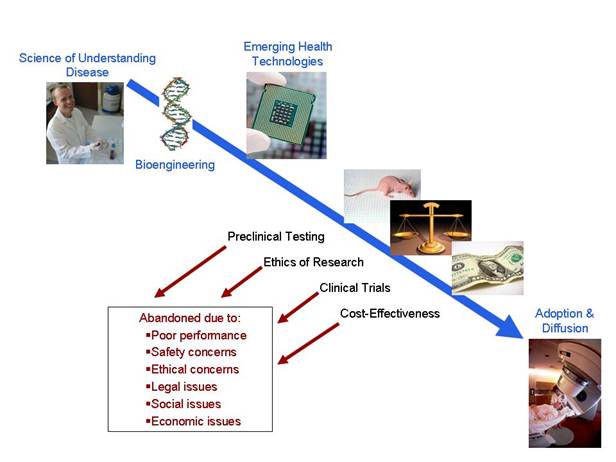
Course Description: This course provides an overview of contemporary technological advances to improve human health. We will consider four questions throughout the semester:
1. What are the problems in healthcare today? 2. Who pays to solve problems in healthcare? 3. How can we use science and technology to solve healthcare problems? 4. Once developed, how do new healthcare technologies move from the lab to the bedside?
We will compare and contrast answers to these questions throughout the developed and developing worlds. We will also consider legal and ethical issues associated with developing new medical technologies. During the semester, we will use case studies to examine a number of diseases and healthcare technologies. Students will complete a semester project to design a new technology to meet a global health need.
NOTE: This course satisfies the Group 3 Distribution Requirement
Prerequisites: Meeting Time and Location: None T/TH 9:25-10:40 AM, Location BRC 282
Course instructor: Yvette Mirabal, MPH BRC Room 174D ymirabal@rice.edu Office Hours: T/TH 10:45-11:45, or by appointment Teaching Assistant: Garrett Spiegel garrett.j.spiegel@vanderbilt.edu
Method of Instruction: The course will consist of lectures and in-class activities. Daily reading assignments will complement material covered in class.
Textbook: The textbook Biomedical Engineering for Global Health can be previewed and purchased at http://www.amazon.com/Biomedical-Engineering-Global-Health-Cambridge/dp/0521877970.
Method of Evaluation: Three in-class exams will be given and will focus on the readings and material covered in lecture. Homework is assigned every few weeks and is due at the beginning of the following class. A comprehensive final exam will cover all material presented throughout the semester.
Grading:
Attendance/Class participation 5% Homework (5% each) 30% Class exams (15% each) 45% Final Exam 20% Total 100%
If you believe that a mistake has been made in grading your homework or exam, you have ONE WEEK to request a regrade in writing. After one week has passed I will not consider any requests to regrade your work.
Attendance/Class participation: A sign-up sheet will be distributed during class. Class participation will be evaluated at the end of the semester.
Homework: Homework assignments are due at the beginning of class on the day specified on the course calendar. Assignments must be submitted in hardcopy form at the front podium. Any assignment turned in after 9:25am will be considered late. Late assignments will have a 50% maximum grade and will only be accepted one week after the assigned due date. Homework should be your own, but general methods of working problems may be discussed with others.
Exams: Three one-hour exams will be given in class throughout the semester, as well as a comprehensive final exam during final exam week as scheduled by the registrar’s office.
Honor System Policy: As with all endeavors at Rice, you are expected to adhere to the Honor Code and follow the guidelines given in the Blue Book. Exams are given under the honor system. Students are encouraged to work together collaboratively on the projects, but each student is expected to contribute an equal share to the final project. Students are encouraged to bring any concerns involving academic integrity to the attention of the instructor. More information can be found at www.ruf.rice.edu/~honor
Disabilities: If you have a documented disability that requires accommodation, please let me know so that we can confidentially discuss your needs. You will also need to register with the Disability Support Services Office in the Ley Student Center.
Course Objectives:
Rice University | Department of Bioengineering Funded by a grant from the Howard Hughes Medical Institute |
 Biomedical Engineering for Global Health
Biomedical Engineering for Global Health