Heat Exchanger Networks
Reducing the Use of Utilities
The use of steam or furnace gases for heating and cooling water for cooling the process streams in a plant is quite inefficient. Reducing the dependence on these has seen tremendous improvements during the last two decades. One procedure for determining how to do this is discussed in some detail in the texts by Douglas1 and Smith2. The following Matlab functions are useful in demonstrating the concepts in those texts.
1Douglas, James, M. Conceptual Design of Chemical Processes,
1988, McGraw-Hill, Chapter 8.
2Smith, Robin, Chemical Process Design, 1995, McGraw-Hill.
Chapters 6 and 7.
| Program | Results in | Assumption |
|---|---|---|
| entHC1 | Returns net enthalpy of one or more streams with different Fcps. | Constant flow*specific heat for each stream. |
| entHC2 | Returns net enthalpy of one or more streams with different Fcps and change of states. | Constant flow*specific heat for each stream except where change of state occurs. Change of state is at constant temperature. |
| entHC | Uses entHC1 and plots results with a specified horizontal shift. | Constant flow*specific heat for each stream. |
| entHCb | Uses entHC2 and plots results with a specified horizontal shift. | Same as for entHC2. |
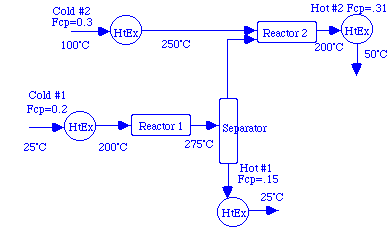
The simple examples that introduce the construction of composite heating and cooling curves in both Douglas and Smith are directly solved using the programs. Here is a rough diagram of a two cold and two hot stream problem similar to those in Douglas and Smith:
|
The following table summarizes the temperatures and flow*heat capacities of the four streams.
| Stream | Inlet Temperature (°C) | Exit Temperature (°C) | Flow*Heat Capacity (MW/°C) |
|---|---|---|---|
| Cold #1 | 25 | 200 | 0.2 |
| Cold #2 | 100 | 250 | 0.3 |
| Hot #1 | 275 | 25 | 0.15 |
| Hot #2 | 200 | 50 | 0.31 |
If we combine the two cold streams (and the two hot streams) we can see that our heating and cooling requirements are:
| Temperature Interval | Cold Streams Flow*Capacity (MW/°C) | Temperature Interval | Hot Streams Flow*Capacity (MW/°C) |
|---|---|---|---|
| 25 to 100°C | 0.2 | 25 to 50°C | 0.15 |
| 100 to 200°C | 0.5 | 50 to 200°C | 0.46 |
| 200 to 250°C | 0.3 | 200 to 275°C | 0.15 |
Following are two examples concerning the analysis of such a system. The first example includes entHC1 and entHC; thus, there is no phase change in the system. The second example includes a phase change, so entHC2 and entHCb are employed.