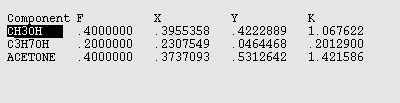 |
This aspect of the CENG 403 page focuses on the use of AspenPlus to separate mixtures of different types taken from examples in BGW. The simulations may be compared to the Matlab versions of the same systems. Here are links to various examples shown in this chapter:
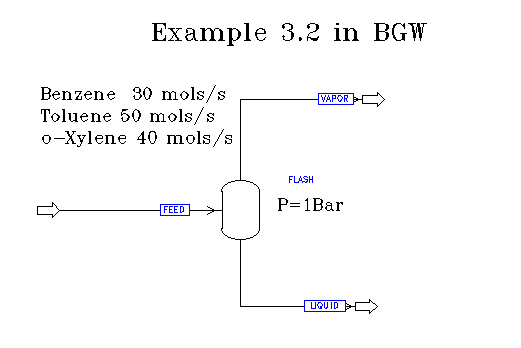
Example 3.2 in BGW:
Example 7.3 in BGW: non-ideal flash at specified T & P
Example 7.4 in BGW: non-ideal flash adiabatic at specified P
Before a simulation may be run, the flow diagram must first be constructed. Below is an image of the first system in Aspen:
 |
The flow diagram was created by the procedure shown in the introductory Aspen section. First the basic diagram was produced to look like:
 |
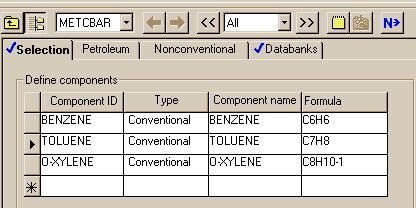
The components were specified for the system.
For this system, we chose the ideal package. Now we need to specify the mole fractions and conditions of the feed stream.
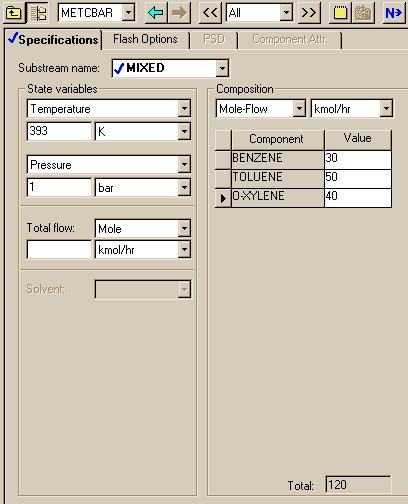
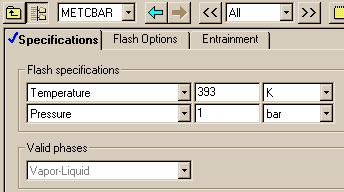
Next we specify the conditions of the flash.
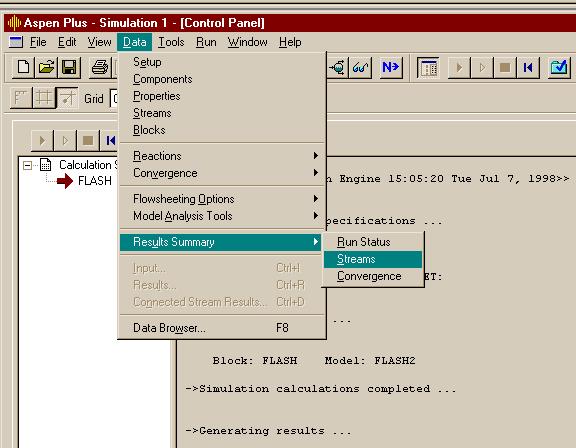
Stream flows and conditions may be seen in the stream summary table that you can get from:
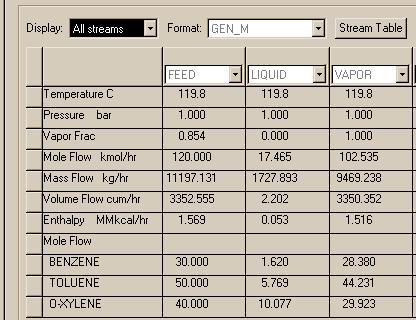
Here is what we found:
If you use the >> button several times, you find results that are normally reported for a flash calculation. Here is an image of that window that shows mol fractions and K values for the streams.
NOTE: In the Aspen results windows, F stands for the FEED stream, X is the LIQUID stream, Y is the VAPOR stream, and K = Y/X.
Flash Conditions: 393 K and 1 bar
That is the basic procedure for performing a flash in Aspen. Now let's see what is required to solve the three types of flash problems in Example 3.2 of BGW. The compounds are as close to being ideal as you are likely to find in a separations problem. Thus the Ideal thermodynamics package was used in all cases.
Case 1: Given the pressure is 1bar and the fraction of the toluene in the vapor should be 90%, find the flash temperature and display the results. This appears to require the use of a sensitiviy run in Aspen to find the temperature. Plotting the flow of toluene in the vapor vs the flash temperature shows:
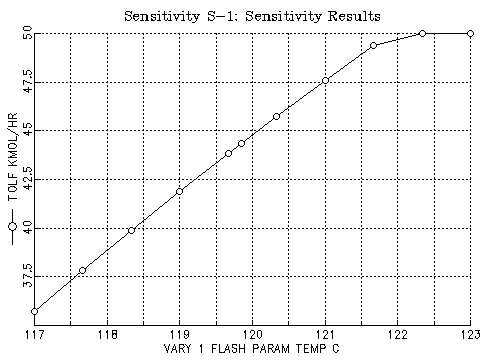
Since the flow in the feed is 50 kmol/hr, we would like to have 45 kmol/hr in the vapor. Thus the temperature: 120.1C should be about right. Here are the results found for this case:
Display ALLSTREAMS FEED LIQUID VAPOR
Units: From FLASH FLASH
Format: GEN_M To FLASH
Phas MIXED LIQUID VAPOR
Temperature [C] 119.8 120.1 120.1
Pressure [BAR] 1.000 1.000 1.000
Vapor Frac 0.857 0.000 1.000
Mole Flow [KMOL/HR] 120.000 15.142 104.858
Mass Flow [KG/HR] 11197.131 1499.754 9697.377
Volume Flow [CUM/HR] 3362.541 1.911 3428.447
Enthalpy [MMKCAL/H 1.572 0.045 1.545
Mole Flow [KMOL/HR]
C6H6 30.000 1.377 28.623
C7H8 50.000 4.933 45.067
C8H10 40.000 8.832 31.168
Mole Frac
C6H6 0.250 0.091 0.273
C7H8 0.417 0.326 0.430
C8H10 0.333 0.583 0.297
You can see that slightly over 45 kmol/hr of toluene were recovered in the vapor.
Case 2: Given P=1bar and T=390K is straight forward leading to the results:
Display ALLSTREAMS FEED LIQUID VAPOR
Units: From FLASH FLASH
Format: GEN_M To FLASH
Phas MIXED LIQUID VAPOR
Temperature [C] 116.8 116.8 116.8
Pressure [BAR] 1.000 1.000 1.000
Vapor Frac 0.664 0.000 1.000
Mole Flow [KMOL/HR] 120.000 40.312 79.688
Mass Flow [KG/HR] 11197.131 3942.939 7254.192
Volume Flow [CUM/HR] 2588.982 5.015 2583.967
Enthalpy [MMKCAL/H 1.365 0.142 1.223
Mole Flow [KMOL/HR]
C6H6 30.000 4.627 25.373
C7H8 50.000 14.760 35.240
C8H10 40.000 20.925 19.075
Mole Frac
C6H6 0.250 0.115 0.318
C7H8 0.417 0.366 0.442
C8H10 0.333 0.519 0.239
Case 3: Given P=1bar and V/F=0.8 allows us to use one of the direct options in the FLASH2 package. Here is the way the specification form should look:
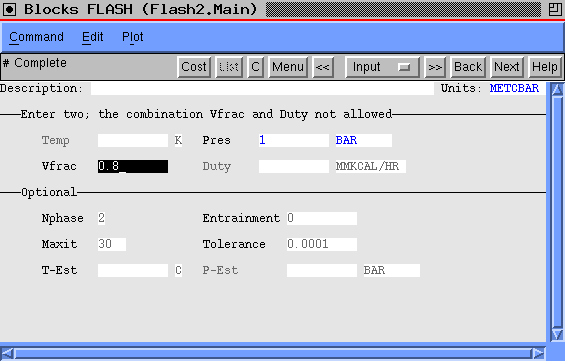
Here is what we then find:
Display ALLSTREAMS FEED LIQUID VAPOR
Units: From FLASH FLASH
Format: GEN_M To FLASH
Phas MIXED LIQUID VAPOR
Temperature [C] 116.8 119.0 119.0
Pressure [BAR] 1.000 1.000 1.000
Vapor Frac 0.664 0.000 1.000
Mole Flow [KMOL/HR] 120.000 24.000 96.000
Mass Flow [KG/HR] 11197.131 2367.096 8830.035
Volume Flow [CUM/HR] 2588.982 3.015 3129.941
Enthalpy [MMKCAL/H 1.365 0.076 1.435
Mole Flow [KMOL/HR]
C6H6 30.000 2.365 27.635
C7H8 50.000 8.169 41.831
C8H10 40.000 13.466 26.534
Mole Frac
C6H6 0.250 0.099 0.288
C7H8 0.417 0.340 0.436
C8H10 0.333 0.561 0.276
Example 7.3 is similar to the previous one, except the compound are definitely non-ideal in the liquid phase. I chose the Van Laar activity model since it sounded like it would be appropriate for this system where the liquid is non-ideal, but the vapor should be close to ideal. Here is the first part of the help message about the activity model:

Here is the flow diagram:
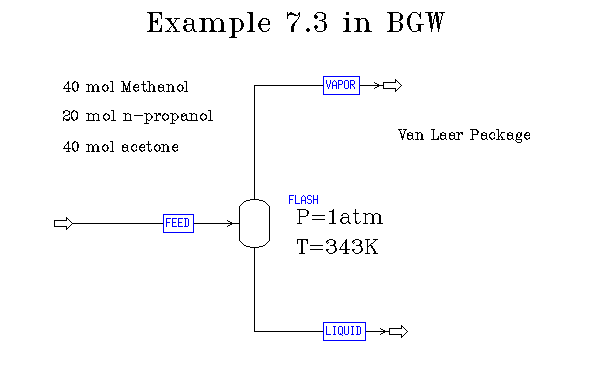
In Aspen there is no more problem in executing a flash for a non-ideal system than an ideal one. Here were the results:
Display ALLSTREAMS FEED LIQUID VAPOR
Units: From FLASH FLASH
Format: GEN_M To FLASH
Phas MIXED LIQUID VAPOR
Temperature [C] 70.0 70.0 70.0
Pressure [BAR] 1.013 1.013 1.013
Vapor Frac 0.613 0.000 1.000
Mole Flow [KMOL/HR] 100.000 38.704 61.296
Mass Flow [KG/HR] 4806.807 1919.045 2887.761
Volume Flow [CUM/HR] 1728.474 2.541 1725.933
Enthalpy [MMKCAL/H -5.462 -2.377 -3.085
Mole Flow [KMOL/HR]
CH3OH 40.000 13.660 26.340
C3H7OH 20.000 13.281 6.719
ACETONE 40.000 11.763 28.237
Mole Frac
CH3OH 0.400 0.353 0.430
C3H7OH 0.200 0.343 0.110
ACETONE 0.400 0.304 0.461
That is not the expected result. Compare these compositions to those found with Matlab. Maybe we should look at some more of the help message about the Van Laar model:

It appears that Aspen does not furnish data for the Van Laar activity model. Let's try a different model. The Wilson model looks promising:

The built-in parameters look good. Here is a simulation of example 7.3 with it as our thermodynamics package:
Display ALLSTREAMS FEED LIQUID VAPOR
Units: From FLASH FLASH
Format: GEN_M To FLASH
Phas MIXED LIQUID VAPOR
Temperature [C] 70.0 70.0 70.0
Pressure [BAR] 1.013 1.013 1.013
Vapor Frac 0.751 0.000 1.000
Mole Flow [KMOL/HR] 100.000 24.894 75.106
Mass Flow [KG/HR] 4806.807 1260.078 3546.728
Volume Flow [CUM/HR] 2116.463 1.663 2114.801
Enthalpy [MMKCAL/H -5.349 -1.555 -3.794
Mole Flow [KMOL/HR]
CH3OH 40.000 7.940 32.060
C3H7OH 20.000 10.405 9.595
ACETONE 40.000 6.549 33.451
Mole Frac
CH3OH 0.400 0.319 0.427
C3H7OH 0.200 0.418 0.128
ACETONE 0.400 0.263 0.445
The VLE Table for this case looks like:
The adiabatic case is no more difficult than normal flash simulations. You just need to specify that the Duty is zero rather than the temperature in the input file for the flash unit. In our example we need to change the feed stream as well to correspond to the conditions given in the problem. Here is a flow diagram with information about the specifications:
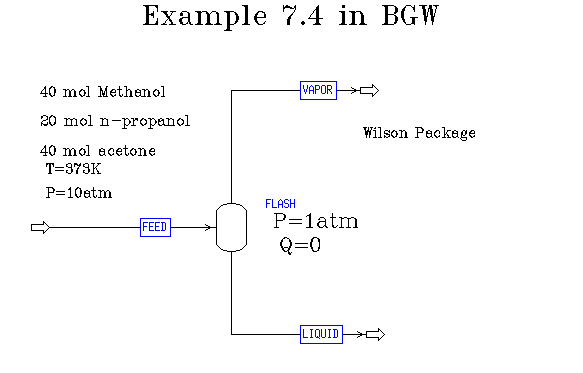
Here is what we find for the results of this flash:
Display ALLSTREAMS FEED LIQUID VAPOR
Units: From FLASH FLASH
Format: GEN_M To FLASH
Phas LIQUID LIQUID VAPOR
Temperature [C] 99.8 63.2 63.2
Pressure [BAR] 10.132 1.013 1.013
Vapor Frac 0.000 0.000 1.000
Mole Flow [KMOL/HR] 100.000 83.313 16.687
Mass Flow [KG/HR] 4806.807 4019.559 787.247
Volume Flow [CUM/HR] 6.785 5.296 460.492
Enthalpy [MMKCAL/H -5.841 -5.009 -0.832
Mole Flow [KMOL/HR]
CH3OH 40.000 32.953 7.047
C3H7OH 20.000 19.225 0.775
ACETONE 40.000 31.135 8.865
Mole Frac
CH3OH 0.400 0.396 0.422
C3H7OH 0.200 0.231 0.046
ACETONE 0.400 0.374 0.531
Finally here is what the VLE form tells us: