TPWS
Thermodynamic
Properties of Water and Steam
by Howard Chao and Stella Unruh
Creating/Specifying the
Unit Set
All
of the calculations found with the IAPWS IF-97 may be converted to any
unit. However, because the possibilities
of different units are endless, only a select few were built into this
application. The header file for tpws_SetUnit.m provides all of the necessary
information for changing the unit set.
The structure for the unit set is shown below.
Code from tpws_SetUnit.m
...
switch
unitSet
% IAPWS Standard
case {'IAPWS','iapws'}
tpwsUnits.T = 'K';
tpwsUnits.p = 'MPa';
tpwsUnits.v = 'm3/kg';
tpwsUnits.e = 'kJ/kg';
tpwsUnits.e2 = 'kJ/kg K';
...
Two
default unit sets are preprogrammed into the application (IAPWS and English
units). However, the user may create
additional custom unit sets to use with the code. However, the desired unit set must be
specified in Line 39 of tpws.m. By
default, all calculations are made using the IAPWS standard units.
Code from tpws.m
...
% Name of desired unit set
unitSet
= 'IAPWS';
% Initialize the data structure
tpwsData
= tpws_IntData;
...
Additional
units may be added to the library by modifying the files tpws_Convu.m and tpws_ConvuStand.m. This process is rather self-explanatory and
only requires a minimal amount of programming knowledge.
Acquiring Thermodynamic
Properties
Once
the unit set has been properly specified, thermodynamic properties of water and
steam are only seconds away! The calling
syntax for tpws.m is given in the header file
(reproduced below).
Code from tpws.m
...
%
Syntax: [tpwsData, tpwsData2] =
tpws(p,T,dispTable)
% p Pressure [MPa]
% T Temperature [K]
% dispTable Optional Parameter to disable the table
% display of
thermodynamic properties. By
% default, the
table feature is enabled.
% 0 = Disable
table display
% Note: No
action necessary to enable
% table
display
%
-------------------------------------------------------------------------
%
Output: tpwsData Data structure for the properties of water
% tpwsData2 Data structure for the vapor phase
% when the water
is saturated (otherwise, no
% data is stored
in this parameter)
...
For
instance, to determine the properties of water at (5 MPa, 500 K), simply enter
the following command into the Matlab command window
>>
data1 = tpws(5,500);
Thermodynamic
Properties of Water and Steam
-----------------------------------------------------------------------
Temperature 500.00000000 K
Pressure 5.00000000 MPa
Specific
Volume 0.00119973 m3/kg
Internal Energy 969.98873381 kJ/kg
Enthalpy 975.98739961 kJ/kg
Entropy 2.57650516 kJ/kg K
Isobaric
Heat Capacity 4.63842045 kJ/kg
K
Isochoric
Heat Capacity 3.21984210 kJ/kg
K
State Liquid
-----------------------------------------------------------------------
The
data values generated by this code are stored in a data structure. The names of each field are shown below. The code below also shows how to access these
fields values individually.
>> data1
data1 =
T: 500
p: 5
v: 0.00119973315866
u: 9.699887338129248e+002
s: 2.57650515860174
h: 9.759873996062026e+002
c_p: 4.63842044505813
c_v: 3.21984209760407
state: 'Liquid'
>> data1.v
ans =
0.00119973315866
For
instance, to calculate the difference in enthalpy between water at (5 MPa, 500
K) and (5 MPa, 530 K) we would enter the following
>>
data2 = tpws(5,530,0);
>>
data2.h – data1.h
ans
=
1.431806415316273e+002
Notice
that by passing an additional parameter through the calling sequence we have
effectively disabled the table display (0 = disable). This may be useful in saving computational
time if displaying the table becomes unnecessary.
In
order to determine the properties of saturated water, we may make use of the
functions region4_pcalc.m and region4_Tcalc.m.
The following code shows how to find saturated properties at a given
temperature.
>> T = 300;
>>
tpws(region4_pcalc(T), T);
Thermodynamic Properties
of Water and Steam
-----------------------------------------------------------------------
Temperature 300.00000000 300.00000000 K
Pressure 0.00353659 0.00353659 MPa
Specific Volume 0.00100350 39.08205832 m3/kg
Internal Energy
112.57144185 2411.67581460
kJ/kg
Enthalpy
112.57499081 2549.89300831
kJ/kg
Entropy
0.39312360 8.51753669 kJ/kg
K
Isobaric Heat
Capacity 4.18137309 1.91393268 kJ/kg K
Isochoric Heat
Capacity 4.13100779 1.44209965 kJ/kg K
State Liquid Vapor
-----------------------------------------------------------------------
Likewise,
to find saturated water properties at a given pressure use the following sequence.
>> p = 5;
>>
tpws(p,region4_Tcalc(p));
Thermodynamic Properties
of Water and Steam
-----------------------------------------------------------------------
Temperature 537.09287119 537.09287119 K
Pressure 5.00000000 5.00000000 MPa
Specific Volume 0.00128641 0.03944627 m3/kg
Internal Energy
1148.07001428 2596.99571006
kJ/kg
Enthalpy
1154.50204233 2794.22706605
kJ/kg
Entropy
2.92074596 5.97370405 kJ/kg
K
Isobaric Heat Capacity 5.03218240 4.43783773 kJ/kg K
Isochoric Heat
Capacity 3.11517459 2.59046427 kJ/kg K
State Liquid Vapor
-----------------------------------------------------------------------
However,
when using these commands you MUST be operating under the default IAPWS units
(MPa and K).
When performing further calculations involving saturated
properties, be sure to pass two data structures to tpws.m
because there are two sets of computed thermodynamic data!
>> [liquid, vapor]
= tpws(region4_pcalc(373.15), 373.15);
Thermodynamic Properties
of Water and Steam
-----------------------------------------------------------------------
Temperature 373.15000000 373.15000000 K
Pressure 0.10141798 0.10141798 MPa
Specific Volume 0.00104346 1.67186060 m3/kg
Internal Energy
418.99332986 2506.01530769
kJ/kg
Enthalpy 419.09915500 2675.57202922 kJ/kg
Entropy
1.30701433 7.35407705 kJ/kg
K
Isobaric Heat
Capacity 4.21664512 2.07749187 kJ/kg K
Isochoric Heat
Capacity 3.76770022 1.55369870 kJ/kg K
State Liquid Vapor
-----------------------------------------------------------------------
Thus,
the enthalpy of vaporization at 373.15 K (100°C) may be easily
calculated using the following command.
>> vapor.h - liquid.h
ans =
2.256472874223131e+003
Slightly Advanced Topics
Because
all of the calculated data may be accessed individually and stored into an
array, thermodynamic plots become incredibly easy to construct. For example, the following code will plot the
isobaric heat capacity as a function of temperature at constant pressure under
the conditions
300 K ≤ T ≤
1000 K p = 10 MPa.
clear
% Array
index counters
k = 1;
j = 1;
% Starting
conditions
p = 10; % MPa
T = 300; % K
while (T < 1000)
% Acquire Data using tpws (disable table
display)
tpwsData = tpws(p,T,0);
% Store the
computed data into proper arrays
switch tpwsData.state
case {'Liquid'}
TdataL(k) = T;
cpdataL(k) = tpwsData.c_p;
k = k + 1;
otherwise
TdataV(j) = T;
cpdataV(j) = tpwsData.c_p;
j = j + 1;
end
% Increment the
temperature
T = T + 8;
end
% Plot
results
plot(TdataL, cpdataL, '-b.',
TdataV, cpdataV, '-r.');
xlabel('T [K]');
ylabel('C_P [kJ/kg K]');
grid on;
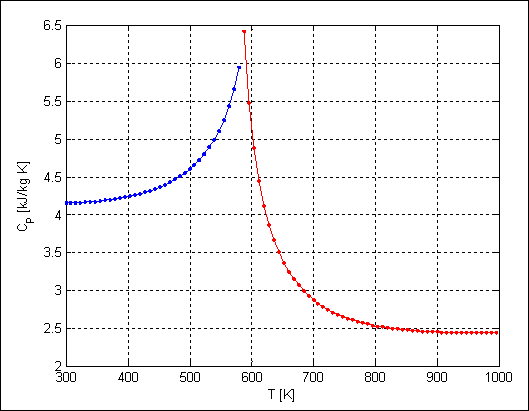
A
similar plot was obtained from NIST using the same
conditions.
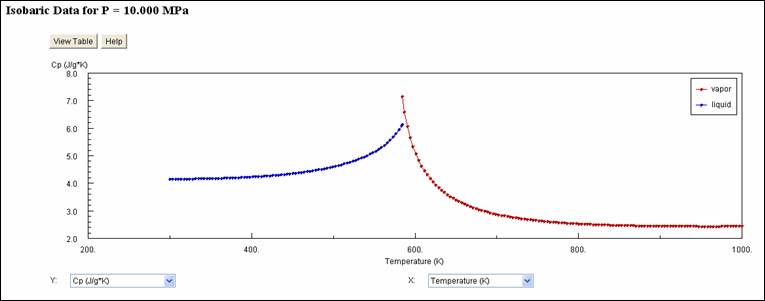
From
visual inspection the results from both sources are equivalent! This example illustrates one of the many
powerful capabilities of tpws.m.
[ Introduction | Matlab
Code | Program
Verification | Examples | Suggested Improvements | References ]