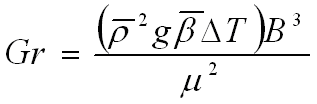
For an ideal gas Beta is 1/T. The temperature is assumed to be linear. This implies that the average temperature is the arithmetic average of the two extreme temperatures.
Grashof Number for an Ideal Gas
| Most of the fluids
dealt with in ChBE 402 are ideal gases. The equation for the Grashof Number
simplifies for the case of an ideal gas.
For an ideal gas Beta is 1/T. The temperature is assumed to be linear. This implies that the average temperature is the arithmetic average of the two extreme temperatures. |
MATLAB Code
| function
Grashoff = grigcalc (Th,Tc,P,B,index) % Calculates the Grashoff number assuming the ideal gas law % holds for the system. Note: For Bird, Stewart and Lightfoot % parameter 'B' should be the radius of the tube or half the % distance between two parallel plates % Argument List: % Th [=] temperature of the hot plate in units of Tdeg % Tc [=] temperature of the cold plate in units of Tdeg % P [=] pressure in units of Pa % B [=] the representative length of the system in units of m % index [=] index of compounds whose Grashoff numbers are calculated % Returns: % Gr [=] Grashoff number in dimensionless units % Example: % for a single component system of water (Tdeg=Kelvin) % >> grigcalc(500,350,101325,0.01) % ans = % 4.2786e+003 Thabs=at(Th); Tcabs=at(Tc); deltaT=abs(Thabs-Tcabs); beta=2/(Thabs+Tcabs); if nargin<4 fprintf('Too few arguments') elseif nargin==4 rhobar=rhocalc((Th+Tc)./2,P); Grashoff=rhobar.^2.*9.8.*beta.*deltaT.*B.^3./(mucalc((Th+Tc)./2)).^2; else rhobar=rhocalc((Th+Tc)/2,P,index); Grashoff=rhobar.^2.*9.8.*beta.*deltaT.*B.^3./(mucalc((Th+Tc)./2,index)).^2; end |
Prashant Setty, 2005