SEPARATION DESIGN
CENG 403 PROJECT 2
FALL 1999
PRESENTED BY:

ARMADILLO ENGINEERING
(pronounced ar-mah-dee-oh)
Amit Shashi Mistry
Stephanie Michelle King
Christopher Steven Paxton
SEPARATION DESIGN
CENG 403 PROJECT 2
FALL 1999
PRESENTED BY:

ARMADILLO ENGINEERING
(pronounced ar-mah-dee-oh)
Amit Shashi Mistry
Stephanie Michelle King
Christopher Steven Paxton
Table of Contents
ABSTRACT
The purpose of this project was to design an ethylene oxide (EO) separation process to yield 99.5 % purity product from the reactor effluent of the first part of the process. Armadillo Engineering successfully produced 99.7 % pure EO with a 99.94 % yield. Our process design consisted of an absorption column, a water removal system, a light end removal column, and a refining column. Operating conditions were kept at safe levels and the process was optimized to produce desired purity levels and high yield. The process was also optimized to reduce reboiling costs, condensing costs and capital costs of equipment to achieve an economically favorable process design. Aspen's NRTL package was used to model data regressions and model simulations, as well as perform sensitivity runs used to optimize the process. Further investigation into this process would include better vapor-liquid equilibrium data, decreased cooling by the light ends fractionator, and lower reboiler duties in the first water stripper.
EO is produced through the partial catalytic oxidation of ethylene. Undesirable side reactions produce significant amounts of carbon dioxide and small amounts of acetaldehyde and formaldehyde. Unreacted ethylene and oxygen, inert gases, and water are the other contaminants to be removed in the purification process. This proposal presents an economically favorable, optimized EO separation process design yielding 99.5 % purity ethylene oxide.
The physical properties of ethylene oxide relevant to separation were also taken into consideration in this proposal. The boiling point of EO is 50.8 ºF, which is fairly close to the boiling points of two contaminants in the process, acetaldehyde (69.53 ºF) and formaldehyde (-2.38 ºF). These components were also the most difficult to separate and were left to the final portions of the process to remove.
Due to the especially high reactivity of EO and the high risk of explosion, extra precautions were made to ensure safe operation. The auto-ignition temperature of EO (804 ºF at 1 atm) was carefully avoided. In fact, the highest temperature and pressure in our process were 482 ºF and 241 psi, respectively. These conditions occurred only in the reactor effluent, which was out of our control. The material used for construction was carbon steel for the vessel and stainless steel for the trays. The carbon steel was chosen to lower costs and the stainless steel was chosen to avoid rust contamination, which may cause an undesirable polymerization reaction.
Aspen was chosen to model this process because of its advanced data
regression capabilities. Within Aspen, several thermodynamic packages were
tested. In the end, the NRTL package was chosen over UNIQUAC and Wilson
because of improved results and better regressions. To further improve
the reliability of our model, certain modeling options were utilized.
Data Regression
Aspen's parameters for modeling vapor-liquid equilibrium (VLE) are not always reliable. Therefore, data regression analysis was used to determine whether Aspen could successfully model the binary vapor-liquid systems involved in our process. VLE data was found for the following systems: 1) ethylene oxide/water, 2) formaldehyde/water, 3) acetaldehyde/water, and 4) ethylene oxide/acetaldehyde. Aspen used thermodynamic packages to model data and these results were compared to the default model results produced by Aspen. The NRTL, Wilson, and UNIQUAC packages were used to model the data. Results from this analysis can be seen in Figures R1-R3. Only NRTL results are shown since that was the final package chosen based on good quality modeling. Wilson results were not shown due to poor quality of results. UNIQUAC results were only slightly worse than the NRTL results. The ethylene oxide/water system (Figure R-1) was the only system where Aspen's default NRTL estimation deviated from the data regression. Therefore, the default binary interaction parameters for this system were replaced in subsequent modeling. The acetaldehyde/water system showed good regressions matching well with default values, and thus its binary interaction parameters were not altered. The formaldehyde/water system's analysis (not shown because of large error) also showed that the default data was not accurate, however, the regression also deviated from the experimental, and was thus not used. The same is true for the acetaldehyde/EO system shown in Figure R-3.
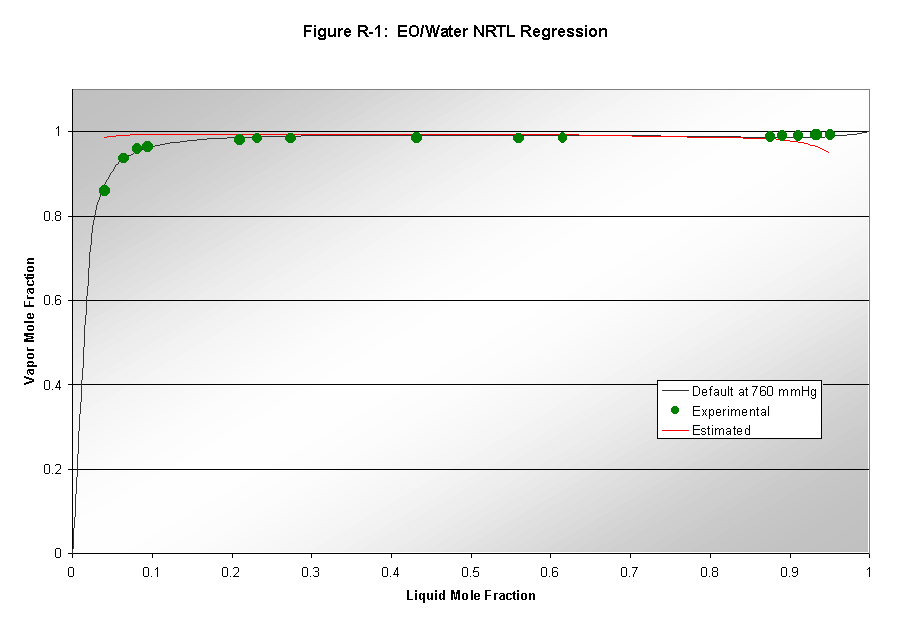
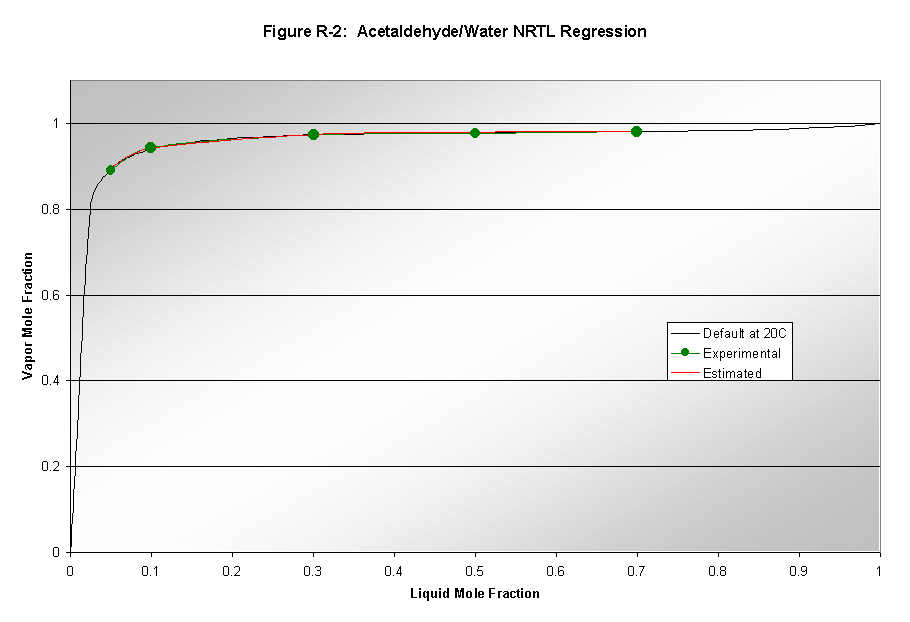
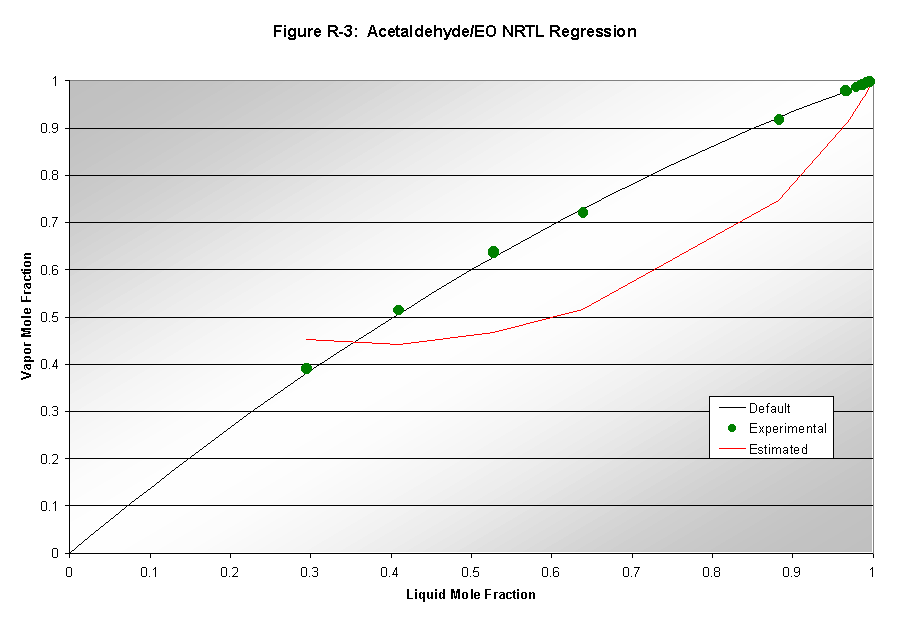
Our process contains numerous gases at temperatures above their critical temperatures. For Aspen to correctly model these components, they were set to be Henry components. The compounds chosen to be Henry components are given in Table A-1 with each compound's critical temperature.
Table A-1: Henry Components
| Henry Component | Critical Temperature |
| CO2 | 87.7 F |
| O2 | -181.38 F |
| N2 | -232.53 F |
| Argon | -188.39 F |
The first unit in the process, the absorber, operates at a much higher temperature than the highest temperature listed above. Methane, ethane, and ethylene also had low critical temperatures and could also have been made Henry components, but were not because of errors during Aspen runs. The errors may have resulted due to the fact that these components were hydrocarbons. Making the compounds listed in Table A-1 Henry components made our final results more reliable and made the CO2 separation more realistic in modeling.
Figure F-1: Overall Process Flow Diagram
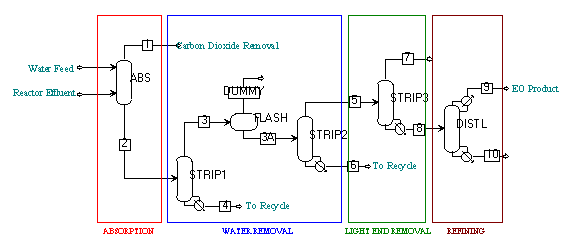
Figure F-1 shows the overall process flow diagram decided upon by our group. The separation process was divided into sections based on the primary separation occurring in that stage. The sections were absorption, CO2 removal, water removal, gas removal, and refining. Each stage was optimized individually taking performance and economics into account. The overall process was also optimized to lower overall costs while maintaining yield and purity. Optimizations were done through sensitivity analyses in Aspen. First, each unit was optimized for yield and cost separately. The units were then combined and slight adjustments were made to further optimize the process. The graphs shown in this section represent sensitivity analyses where the stated variables were adjusted while the other input specifications were held at their optimal value. The specifications that were used in our optimal process are shown as green circles. This technique shows the optimization of the process as a whole by showing that any deviation from the optimal inputs would result in a less optimal process.
Feed Stream
The composition of the feed stream into the separation process was obtained from Say It Ain't So, EO & Sons CENG 404 Project Report and the feed flowrate and conditions were that of jEO & Associates CENG 403 Project 1 Report.
Our goals in modeling the absorber were to remove as much CO2 from the reactor effluent as possible while not losing a significant amount of EO. Although economic considerations were taken in minimizing the number of stages in the column, the majority of the optimization involved the quality of separation. A single absorption column was used with a large feed of water to separate CO2 from the stream. The relatively high solubility of EO in water allowed for good adsorption into an EO-rich stream. This stream continued on to the water stripping section while the gas stream from the top of the column went to the CO2 removal system. The amount of water fed to the unit was the main variable that was optimized. The number of stages was then minimized. The pressure profile in the column was also specified. A pressure in the top stage that was slightly less than that of the feed was found to be appropriate for all of the columns to ensure reasonable reboiling and condensing temperatures.
Optimizing Water Flowrate
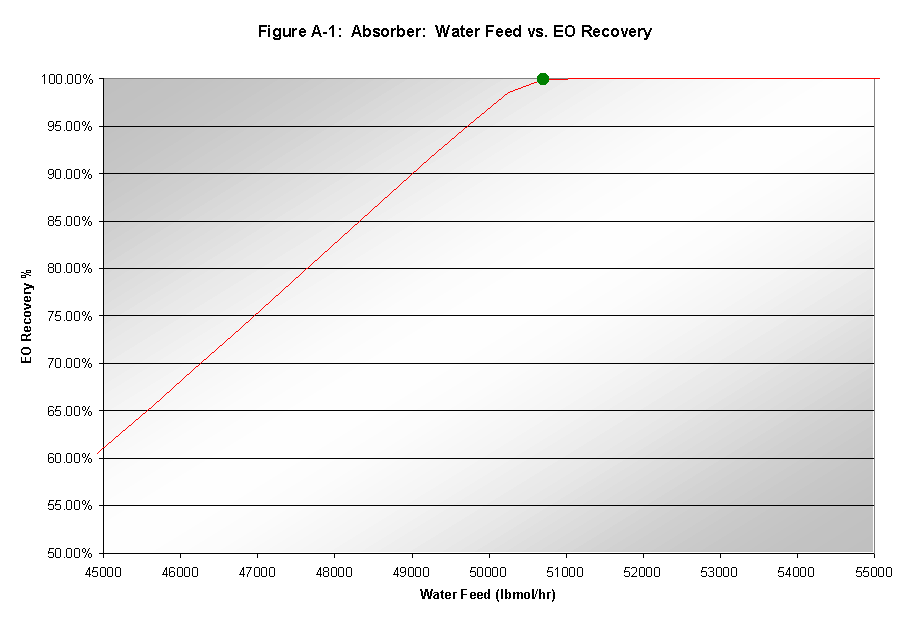
A large flow of water was necessary to achieve the desired adsorption. However, this flow was also kept as low as possible to minimize reboiling duties and thus heating costs of subsequent units. As seen in Figure A-1, a water flowrate of approximately 51,000 lb mole/hr allowed virtually all of the EO to be recoverd.
The residual CO2 in the EO-rich stream increased with any increase in water flowrate. The increase was not significant enough to require any extra reduction in water flow below that which was found optimal for EO recovery.
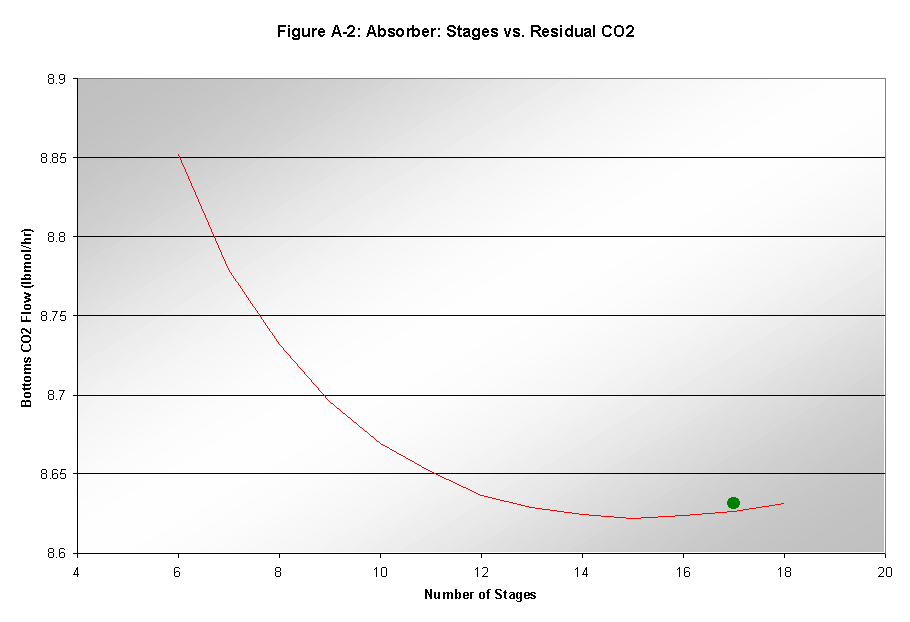
Number of Stages
The number of stages had only a small effect on the EO recovery and residual CO2. Increases in the number of stages did increase the EO recovery, but only by about 0.2% for every added stage. Residual CO2 reached a minimum at approximately 17 stages. This factor, shown in Figure A-2, led to the choice of 17 stages as the optimal input.
It should be noted that the number of stages reported here is the theoretical number of stages based on columns operating at 100% efficiency. In reality, these columns would operate with much more stages. Cost analysis was based on 33% efficiency, which resulted in 51 actual stages. For the purposes of this proposal, number of stages will refer to the ideal number of stages assuming 100 % efficiency except in cost analysis. The absorption column removed 99.56 % of the CO2 and retained 99.97 % of the EO fed into it.
The carbon dioxide removal system was not modeled in Aspen since it is not pertinent to the purification of EO. However, a brief description of the process follows. The scrubbed gas stream leaving the absorber is compressed and a fraction of it is recycled back to the main reactor. Most of the gas stream is sent to the CO2 removal system and the remaining gas is purged to prevent buildup of process inerts, such as nitrogen and argon. The CO2 entering the CO2 absorber is removed using a hot aqueous potassium carbonate solution. Some ethylene is recovered from this and the remaining CO2 is sent to a desorber and vented to the atmosphere. The remaining gases are recycled to the reactor (McKetta 290).
The EO-rich stream leaving the absorber was sent to a series of water strippers, or desorbers, to remove water. Industry examples commonly use one stripper to remove water. The Armadillo Engineering design is unique in that two columns were used. Our process was designed this way to remove a higher percentage of the water and to decrease the reboiling requirements of the water removal by distributing it over two strippers. From Figure F-1, the two strippers were separated by a flash unit used to liquefy the distillate of the first stripper. Each column was optimized individually to lower the reboiling duties with good removal of water and retention of EO in the distillate stream.
Stripper 1
The number of stages was set at 10 and not varied much as it did not have a great effect on the water removal or reboiling duty. This column separated out 91.45 % of the water fed into it.
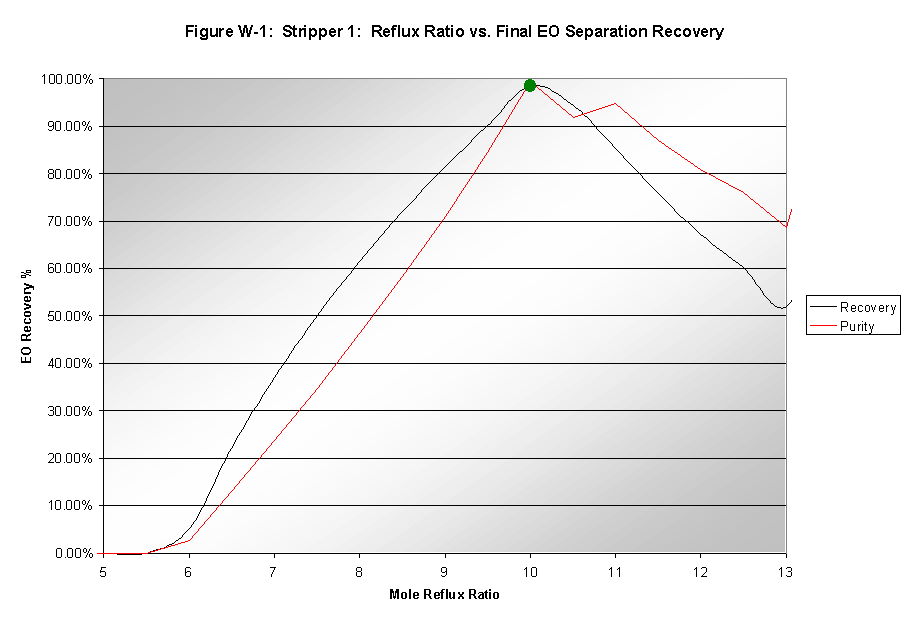
Reflux Ratio
The reflux ratio, however, had a significant effect on water removal. As seen in Figure W-1, the optimal reflux ratio of 10 resulted in very high purity and recovery EO in the final product stream. The reboiler duty at this reflux ratio was also lowered to 238.60 MMBtu/hr. However, this reduction was mostly due to the reduced water flow into the absorber.
Stripper 2
The flash unit preceding the second stripper operated at 217.56 psi and successfully liquefied the vapor distillate from stripper 1. The second water stripper was optimized similar to the first stripper. This unit also operated at 10 stages with very little change in results from increasing or lowering this number. The second column separated out 95.95 % of the remaining water.
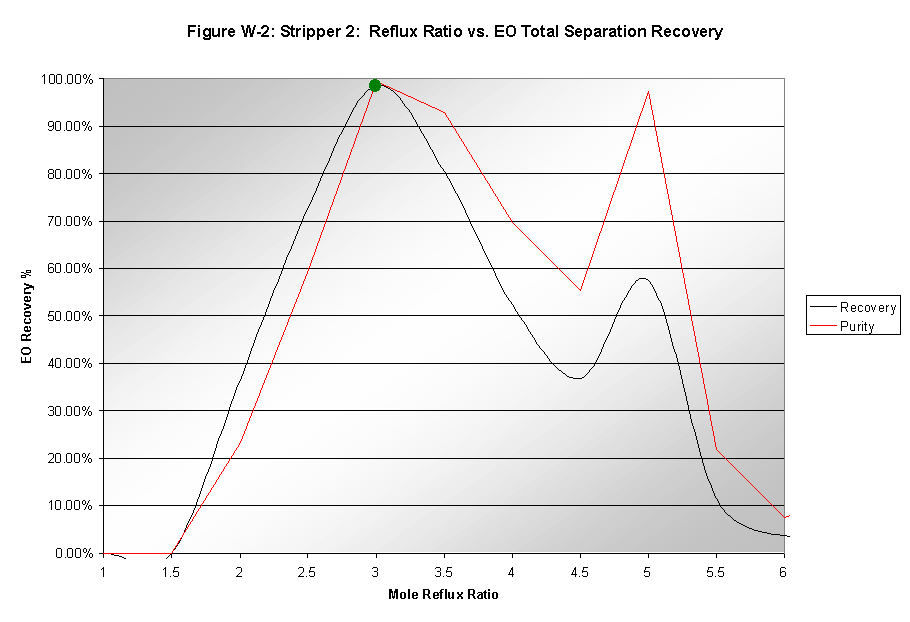
Reflux Ratio
The reflux ratio was optimized to 3 based on EO recovery and purity, as seen in Figure W-2. This value was significantly lower than that of the first column since less water needed to be removed in this step of the process.
A negligible amount of EO was lost in the either of the columns and no EO was lost in the flash unit.
The next step in the purification process was the removal of light end gases such as methane, ethane, and ethylene through a gas fractionator. As in the two water strippers, reflux ratio and the number of stages were considered. Additionally, a partial condenser was used in the light ends removal unit, requiring another specification, the bottoms-to-feed ratio. The feed stage was also considered in the optimization.
Number of Stages and Feed Stage
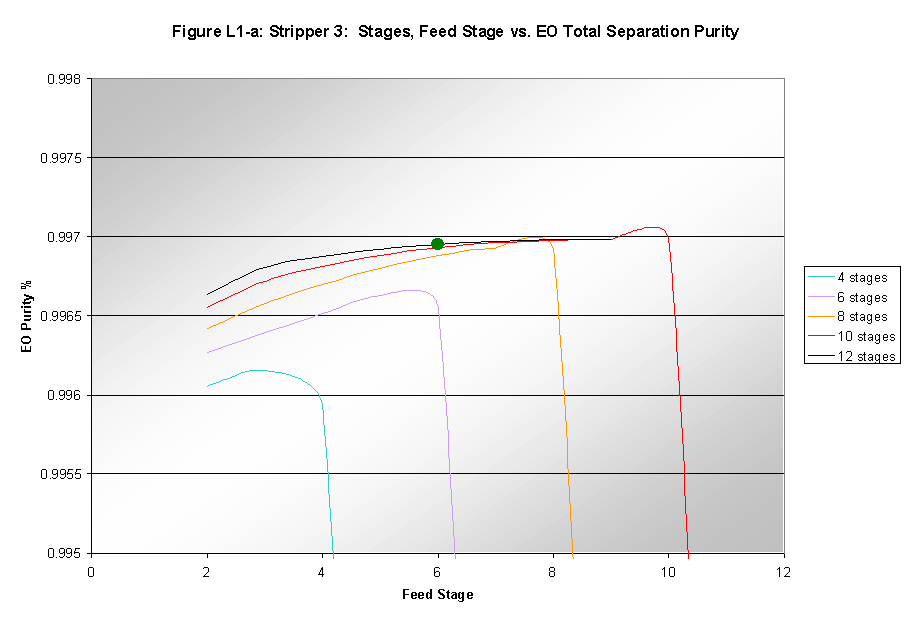
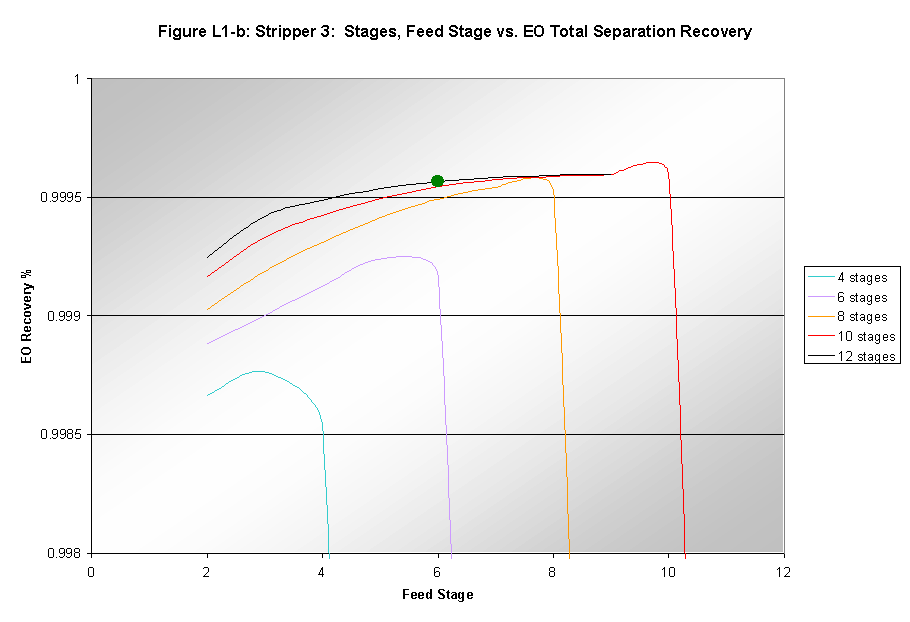
Increasing the number of stages in the light end fractionator did improve the separation of EO from the light end gases. This can be seen in Figure L-1a that shows the purity of EO in the product stream. Figure L-1b shows the reaction of the recovery of EO in the entire separation process to changes in the number of stages and the feed stage.
When the number of stages was increased beyond 12, errors occurred in the simulation due to stages being "dried up." The number of stages was therefore set to twelve. With 12 stages the feed stage had little effect on either the purity or the recovery. The feed was chosen to go into the middle of the column to avoid any unnecessary instability.
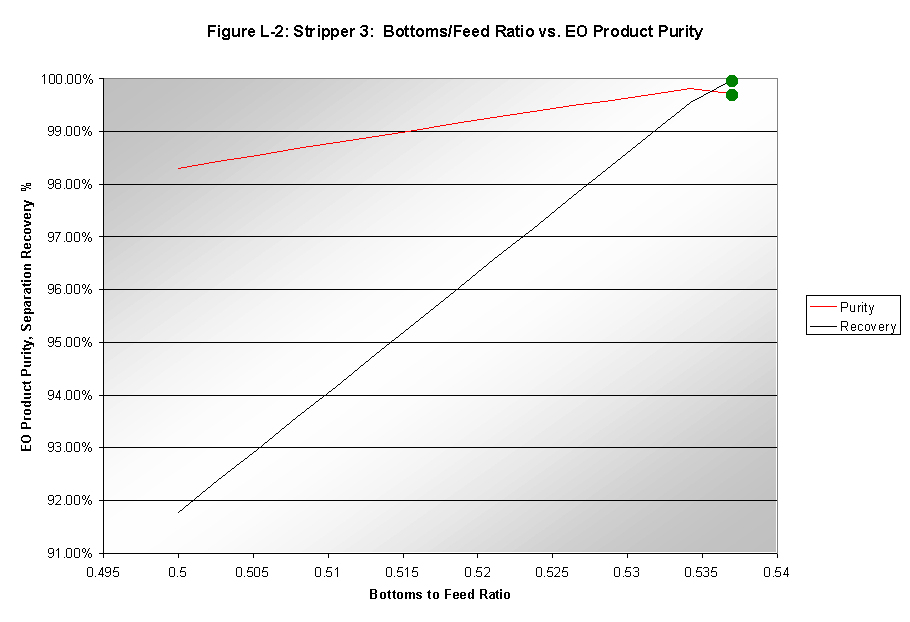
Bottoms-to-Feed Ratio
The bottoms-to-feed ratio (B:F) had a significant effect on the purity and recovery of EO in the product stream. Increases in purity and recovery were observed when the B:F was increased. However, stages "dried up" when the B:F was increased much past 0.538. This system is shown in Figure L-2. The B:F was therefore chosen to be 0.537.
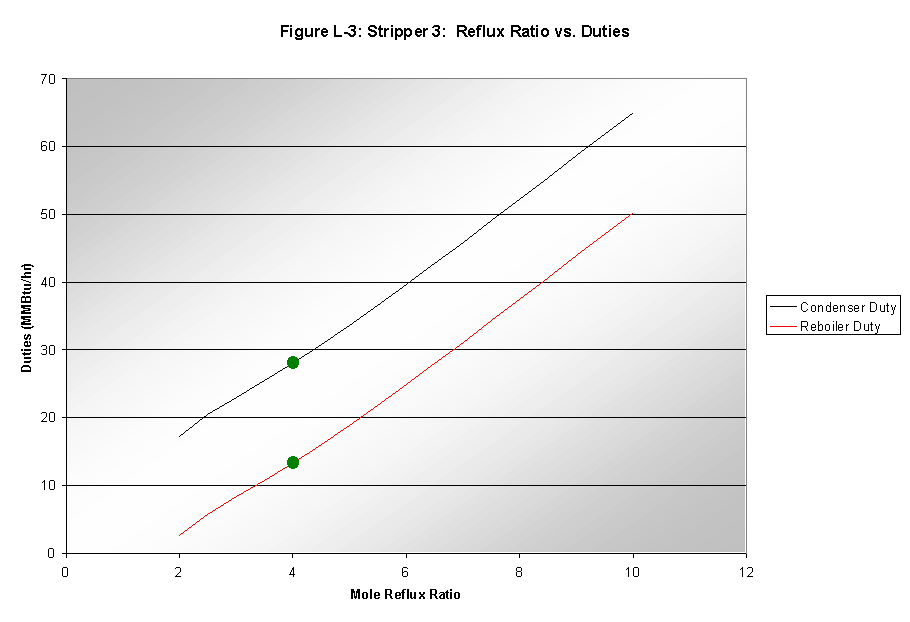
Reflux Ratio
The reflux ratio in the column effected only the duties of the condenser and reboiler, and changes in the reflux were the only specifications that changed these duties significantly. A linear increase in duties resulted from increases in the reflux ratio, as seen in Figure L-3.
A reflux ratio of 4 was chosen as a stable, middle value with reasonably low duties in both the reboiler and condenser.
The light end fractionator retained 99.997% of the EO fed to it, and removed essentially 100% of the ethylene, oxygen, nitrogen, ethane, methane, argon, and carbon dioxide. Trace amounts of formaldehyde and acetaldehyde and a large amount of water remained in the EO stream.
The final product was obtained using a distillation column to separate small amounts of water and smaller amounts of acetaldehyde. The key parameters for this column's optimization were feed stage, number of stages and distillate to feed ratio. The goals of optimization were to reach the desired purity with as high an EO recovery as possible.
Number of Stages and Feed Stage
Figures D-1 and D-2 show the optimization of feed stage location and the number of stages. An increased number of stages results in higher purity and recovery of EO. In addition, higher feed stages up to stage 3 increase EO purity and recovery. Note that Aspen's convention for stage number is from top to bottom. The optimized model used a feed stage in the middle of the tower since the improvement in purity and recovery was not worth risking stability of the tower. The optimal feed stage was stage 10 out of 20 total stages.
Distillate to Feed Ratio
The final parameter in the distillation tower optimization was the distillate to feed ratio (D:F). Decreasing this ratio caused the EO recovery to drop significantly while increasing the ratio caused the EO purity to drop heavily. Thus, as Figure D-3 shows, an optimal value for D:F was at 0.82.
The distillation tower removed all of the remaining water in the sample leaving only trace amounts of acetaldehyde and formaldehyde in the final pure stream of EO.
The economics and optimized profit of the analysis prove that the separation process is favorable and profitable. Certain values in the economic analysis were taken from jEO & Associates EO reactor project to portray a more realistic cost analysis. These values were bare-module equipment costs, feed costs, catalyst costs, and utility costs.
Equipment Costs
The capital costs of the equipment were determined using CAPCOST. Each unit's cost and sizing are given in Table C-1. To size columns, the diameter of each was determined using the tray sizing feature in Aspen. Other considerations taken into account for costing equipment included column efficiency. Heuristics state that columns operate at 33% efficiency
Table C-1
| Equipment | Diameter (ft) | # of Trays | Base Cost |
| Absorber | 16.82 | 51 | $102,326 |
| Stripper 1 | 14.90 | 30 | $64,194 |
| Stripper 2 | 6.32 | 30 | $16,543 |
| Flash | - | - | $50,431 |
| Stripper 3 | 12.95 | 36 | $49,783 |
| Distillation Column | 10.82 | 60 | $44,619 |
| Condenser (2) | - | - | $100,820 each |
| Reboiler (4) | - | - | $24,423 each |
| Total | - | - | $627,228 |
The bare module cost from jEO & Associates was $9,300,000 and the
total bare-module cost found by the method in Appendix C-1 was $12,401,000.
Product Sales and Feed Costs
The breakdown of feed costs from jEO & Associates is shown below
from jEO & Associates
Annual Costs:
Ethylene $59,300,000
Oxygen $4,000,000
Product Sales from Armadillo Engineering
Ethylene Oxide
Price:
$1.15 / kg
Annual Sales:
$148,700,000
Utility Costs
In order to determine the yearly utility costs, it was first necessary to find the mass flow of water and steam given the heat duty and stream temperatures calculated by Aspen. The following equation was used to calculate the amount of cooling water needed by the condensers:
mcw = Qc / [Cp*(Tout-Tin)] ,
where Qc is the condenser duty, Cp is heat capacity, and T is the temperature of the cooling water. Another equation was used to find the amount of steam needed by the reboilers:
mstm = Qr / ?Hvap ,
where Qr is the reboiler duty and ?Hvap is the latent enthalpy of vaporization (970 Btu/lb).
The calculated mass flows and their costs are summarized in Table C-4.
Table C-4: Utility costs per annum
| From jEO & Associates Flow Price Annual Costs | Flow | Price | Annual Costs |
| Water | - | - | $1,800 |
| Electricity | - | - | $870,000 |
| From Armadillo Engineering | |||
| Steam | 360,000 lb/hr | $3.50 / 1,000 lb | $10,054,000 |
| Cooling water | 1,437,000 gal/hr | $0.05 / 1,000 gal | $576,000 |
It is important to note that there is one inaccuracy in the calculation of the utility costs. In the light-ends fractionator, the condenser should be fed with a refrigerated liquid rather than cooling water since the temperature of the stream needs to be around -140°F. However, the price and properties of an appropriate refrigerant could not be found and thus was not analyzed.
One final addition to this analysis is the annual catalyst costs of $3,500,000, which were taken from jEO & Associates. Other operating costs, wages, and miscellaneous expenses are detailed in a cost sheet in Appendix C-2. (Seider 376)
Cash Flow Analysis
The cash flow analysis was based on a two-year construction period, a plant-life of 15 years, and an 8,000-hour operating year. An inflation rate of 1% was assumed for the price of EO, and a 3% rate was assumed for the costs of feed. In order to calculate the net present value, a discount rate of 10% was used in the projected financial analysis. Figure C-1 displays profit over 15 years.
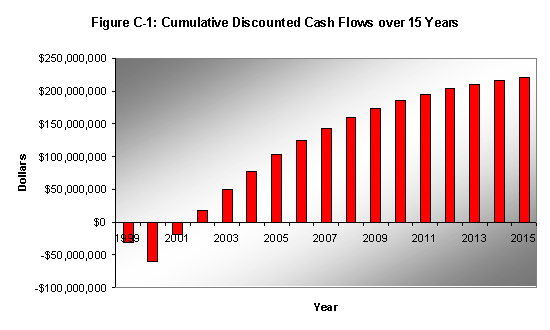
Ethylene Oxide was successfully separated from impurities to 99.5%. The only contaminants in the final product stream were a trace quantity of formaldehyde and acetaldehyde. The yield of EO recovered was 99.94%, with loss of small quantities of EO in the light-end stripper and refining column. The final purity achieved was 99.7%. Other than the high cost of condensing and reboiling, the process is economically viable and profitable.
If given more time to prepare this proposal, Armadillo Engineering would like to have further investigated certain aspects of our design.
The accuracy of our proposal would be much improved with the use of more quality vapor-liquid equilibrium data. The data used in this project were sufficient to model the behaviors of some systems, but other systems were not modeled as well as they could have been because data for these systems was not available. We recommend further investigation into these systems as well as better data for the other systems. The formaldehyde/water system especially could be better modeled using higher quality data.
In addition, the cooling requirements for the light-ends stripper were quite drastic. The distillate needs to be cooled to a temperature of -140 F. The chosen cooler, refrigerated water, does not normally cool to that level, thus other coolants or a process control on this temperature should be investigated.
Finally, the reboiling duty in the first water removal stripper was reduced, but still considerable high. It is recommended that further measures be taken to still lower the flowrate of water or other conditions to decrease these requirements. The high duties result in extremely high costs of steam required to reboil water, thus this is a very important area for further economic optimization.
Anderlik, Jeff. Engineer, Celanese Chemicals and Acetate. Personal correspondence.
Betton, Scott and Chris Ruehl. Ethylene Oxide Purification. CENG 403 Project 2 Report, Fall 1997.
Davis, Samuel H. CENG 403 Professor, Rice University. Personal correspondence.
Ethylene Oxide User's Guide, 2nd edition. Buckles, Carey, et. al. 1996. <http://www.ethyleneoxide.com>.
Flores, Ariel, et. al. Ethylene Oxide Production: 'Say It Ain't' So & Sons. CENG 404 Project 2 Report, Spring, 1999.
Gmehling, J., et. Al. Vapor-Liquid Equilibrium Data Collection. DECHEMA: Frankfurt, Germany, 1979.
Huang, Janet, et. al. Ethylene Oxide Reactor System: jEO & Associates. CENG 403 Project 1 Report, Fall 1999.
McKetta, John J. and Cunnigham, William A. Encyclopedia of Chemical Processing and Design, Marcel Dekker, Inc.: New York, 1983. Volume 20, pp.280-311.
Miller, Clarence A. CENG 403 Professor, Rice University. Personal correspondence.
Seider, Warren D. et,al. Process Design Principles. John Wiley & Sons, Inc.: New York, 1999.
![]()