Table of Contents
Abstract
I. Introduction
II. Description of the Process
III. Economic Optimization
IV. Catalyst
V. Kellogg Advanced Ammonia Process
VI. Conclusion
VII. Endnotes
VIII. References
IX. Appendix
Abstract
Ammonia synthesis optimization is a topic of high interest in
industry as the market continues to expand and demand increases. This
proposed process is designed to produce 1,016 metric tons/day of
ammonia at a feed of 5,500 kmol/hr while maintaining the best
compromise between production and purity. Simulated in ASPEN with an
adiabatic Gibbs reactor, optimal production is achieved at 100 bar
reactor pressure and a 7.25% purge stream, resulting in 98.96%
product stream purity. The simulated process is comparable to
conventional ammonia synthesis plants. Further economic optimization
is focused on compression costs and reactor efficiency. A new
ruthenium-based catalyst with higher activity at lower total
pressures can be employed enabling the process to run at
significantly lower pressures while maintaining high ammonia
conversion. Installing this catalyst into a multi-bed radial
plug-flow reactor results in an attractive combination of high
production and reduced costs that can be custom made for expansion,
retrofit, or grassroots projects.
I. Introduction
The world's first 1000 metric ton per day single train ammonia
synthesis plant was operated by the Mississippi Chemical Corporation
in Yazoo City, Mississippi, USA.
Common industrial ammonia synthesis processes consist of a syngas
feed stream flowing into a compressor and then into a catalytic
converter bed. The effluent from the converter bed enters a heat
exchanger, is cooled, then continues into a separation device. Most
of the product ammonia is removed, while some continues in a recycle
loop with a purge to remove inerts. The recycle stream enters another
compressor then rejoins the input stream into the reactor.
Ammonia is formed from nitrogen and hydrogen by the reversible
reaction given below, and its production is favored by high pressures
and low temperatures.
The objective of this reactor project is to design an ammonia
synthesis plant and find the optimum operating conditions which will
yield 1000 metric tons of ammonia per day.
II. Description of the Process
With respect to the reactor, three questions needed to be addressed
in modeling a typical ammonia synthesis plant:
1. What kind of reactor: plug flow or Gibbs?
2. Under what operating conditions: adiabatic or isothermal?
3. At what specified temperatures and pressures?
The first question, for all practical purposes, was not a choice to
make; the simulation was run with a Gibbs reactor because of
insufficient kinetic data available to execute a plug flow reactor
simulation. This was due to proprietary rights on the catalysts and
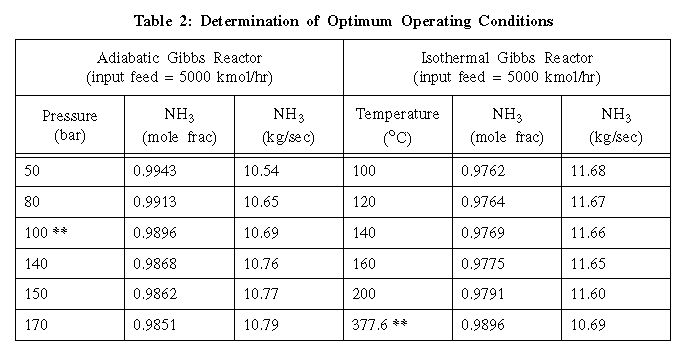
their resulting kinetic data. The remaining two questions were
answered together. In choosing between an adiabatic or isothermal
reactor, pressure was varied in the adiabatic reactor while holding
the temperature of the feed constant, and temperature was varied in
the isothermal reactor while holding its pressure constant. The
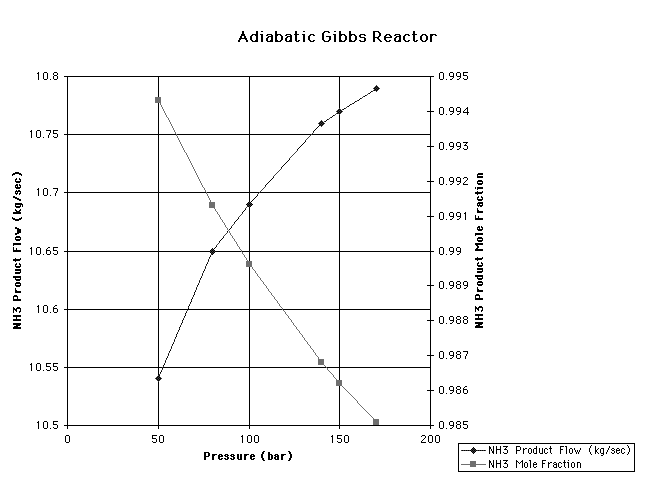
adiabatic Gibbs reactor gave the best results near 100 bar as seen on
the graph entitled "Adiabatic Gibbs Reactor." This is close to the
point of intersection of the product flow line and the purity line,
analogous to the point of equilibrium between supply and demand in
economics. Pressure of 100 bar represented the best trade-off between
the amount of NH3 produced in the product stream and the purity of
the product stream. This pressure was chosen as the constant
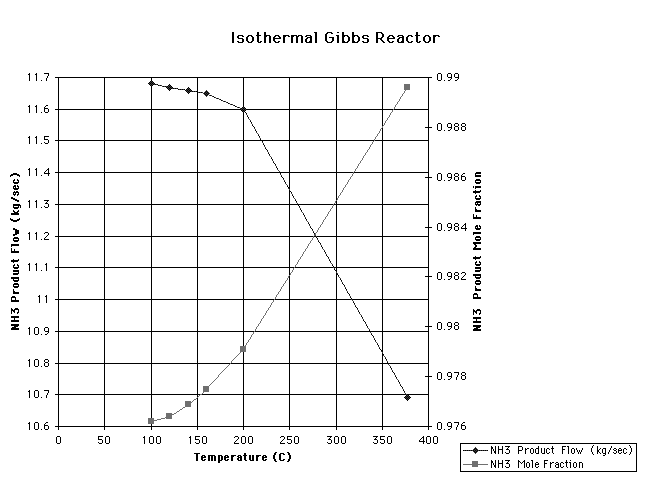
operating pressure of the isothermal Gibbs reactor. The isothermal
Gibbs reactor gave the best results near 275 oC as seen on the graph
entitled "Isothermal Gibbs Reactor." This temperature has the same
implications as the pressure of 100 bar did for the adiabatic Gibbs
reactor.
After obtaining the optimum operating conditions for both the
adiabatic and isothermal reactors, the adiabatic was chosen over
isothermal for two reasons:
1. Adiabatic reactors are more common in industry.
2. It does not require temperature regulation.
The final ammonia plant simulated in ASPEN was based on an adiabatic
Gibbs reactor operated at a pressure of 100 bar and modeled with the
Peng-Robinson thermodynamic package. Peng-Robinson is well suited for
use with gas and hydrocarbon processing and refining. The input feed
stream for the process was 5500 kmol/hr at 40 oC at the following
mole percentages: 74.2% H2, 24.7% N2, 0.8% CH4, and 0.3% Ar.(1) The
product (bottoms) stream from the flash tank produced ammonia at a
rate of 1016 metric tons per day (2486 kmol/hr) at a purity of
98.96%. The recycle purge was operated at 7.25% of the flash tops
stream or 1.49% of the input feed stream. The remaining pieces of the
process flow diagram included two multistage compressors, a heat
exchanger, and a flash vessel. The first compressor was modeled as an
ideal three stage compressor and increased the pressure of the input
feed stream to 100 bar before entering the reactor. The heat
exchanger following the reactor used ammonia (assumed to be produced
by the plant) as the refrigerant to cool down the process stream from
375 oC to -30 oC before entering the flash vessel. The flash vessel
was also operated at -30 oC and had a pressure drop of 5 bar. The
second compressor was an ideal two stage compressor and increased the
pressure of the recycle stream to 100 bar before merging with the
feed stream and entering the reactor.
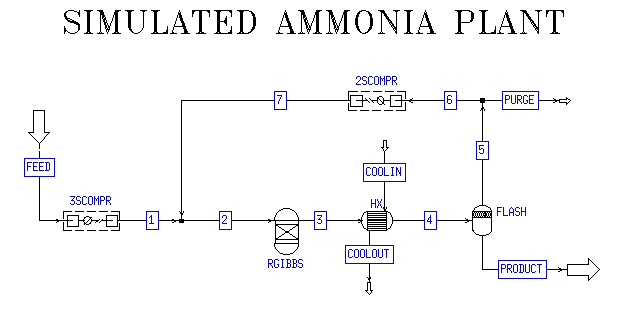
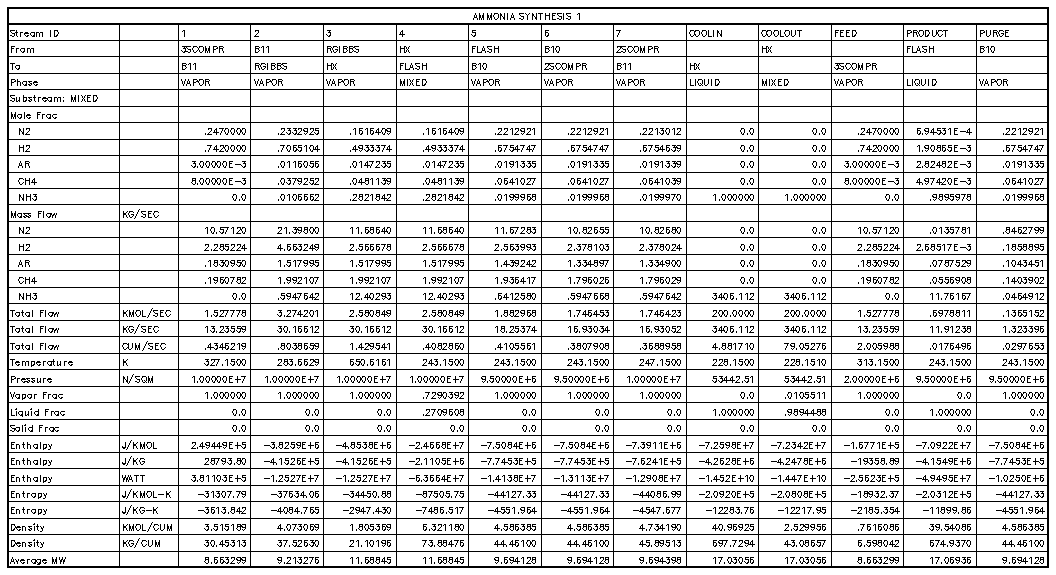
The Haldor-Topsoe Ammonia Synthesis Process(2)
Due to the lack of kinetic data, attempts were made to obtain
physical plant data regarding production rates, reactor layouts and
sizes, and other operating conditions. Plants contacted included
Pacific Ammonia Incorporated in Kitimat, British Columbia, Canada;
Dupont in Beaumont, Texas; and Haldor-Topsoe in Clear Lake, Texas.
The Haldor-Topsoe plant most closely approximated the simulated
ammonia plant. Thus, this plant and its associated data were chosen
as the real world model of the simulation.
The Haldor-Topsoe plant produces over 1500 metric tons per day of NH3
using a two bed converter with internal heat exchangers. This
converter is based on the iron-magnetite catalyst, the most commonly
used today. Their recycle purge operates at 7.26% of the separation
stream or 1.86% of the input feed stream. These numbers strongly
agree with the purges of 7.25% and 1.49% in the simulated plant.
Comparisons of the entrance stream into and exit stream from the
reactors can be made in the following table.
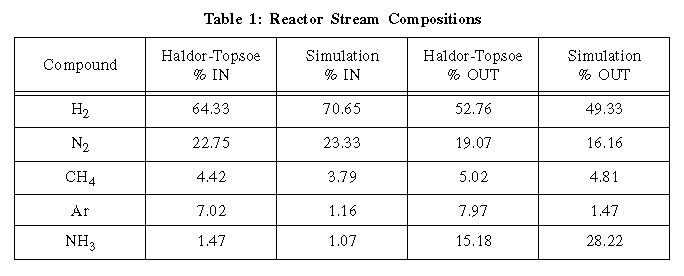
As evidenced, the simulation yielded similar purge percentages and
comparable stream compositions. Slightly higher impurity levels of
the Haldor-Topsoe feed, resulting in catalyst inhibition, may account
for some of the differences. The reactor sizes given were 30 m3 and
75 m3 for the first and second beds, respectively. Of this volume,
approximately 2/3 is taken up by the iron-magnetite catalyst.
III. Economic Optimization
There are a number of means to further optimize these processes.
These are not simulated in ASPEN due to the difficulty in obtaining
detailed quantitative data but are presented here as options that
would result in a more efficient and economically advantageous
process. The primary areas of focus in economic optimization are
compression costs and reactor efficiency. High operating pressures
applied in industrial practice (>100 bar) are usually necessary
for a favorable equilibrium position and high rate of reaction, as
well as ammonia recovery at higher temperatures to reduce
refrigeration costs. However the compression power necessary to
achieve such pressures is one of the most significant expenses in a
plant. A means to reduce this cost is to install centrifugal
compressors driven by steam turbines, equipment used in modern plants
today that take advantage of steam produced elsewhere in the
process.(3) While this is of some advantage, it is not the most
significant improvement that can be made. If reactor efficiency can
be increased such that lower synthesis pressures can be employed
without compromising ammonia conversion, then the costs related to
compression and efficiency can be truly reduced and the process
economically optimized. This can be achieved through the use of a
high activity ruthenium-based synthesis catalyst relatively new to
the market. The catalyst is to be used in conjunction with modern
reactor designs constructed specifically for its optimal
employment.
IV. Catalyst
The industrial process of ammonia synthesis has been generally based
on the reaction of hydrogen and nitrogen at high pressure over a
catalytic surface. Traditionally the catalyst of choice is an
iron-based catalyst with magnetite as its major component. An
attractive alternative to this catalytic system is proposed here as a
means of increasing ammonia conversion at lower pressures, and thus
reducing energy consumption in compressors at a lower capital
cost.
The proposed system utilizes a promoted ruthenium catalyst deposited
on thermally modified active carbon, forming porous cylindrical
pellets about 0.8 mm in diameter and 3-5 mm long, which has been
available to industry relatively recently.(4) This catalyst is up to
twenty times more active than fused iron catalyst at relatively high
conversion degrees. More importantly, although temperature variations
have similar effects on the two catalysts, the effects of ammonia
concentration are significantly different. Iron-based catalyst
activity depends strongly on PNH3 (partial pressure of ammonia). As
PNH3 increases from 1 mol% to 10 mol% the rate of the process
decreases 10 to 25-fold. In contrast, the activity of ruthenium-based
catalysts is only slightly affected by changes in PNH3, as well as
changes in total pressure. Promoted ruthenium catalyst deposited on
active graphite therefore has been found to have excellent low
pressure and low temperature performance.(5) This is of great
importance to industrial practice, taking into account contemporary
tendencies to lower the applied pressure and thus reduce energy
consumption.
Further benefits of using the ruthenium-based catalyst are found in
capital cost savings. As lower pressures are used in the process,
there is greater flexibility in process compressor driver selection.
Thinner-walled and lighter vessels, piping, and fittings can be
employed safely, all of which are equipment more commonly fabricated
world-wide and therefore cheaper. This new catalyst opens a world of
possibilities for industrial ammonia synthesis optimization,
maintaining high ammonia conversion and safety standards at
significantly reduced costs and increased profit.
V. Kellogg Advanced Ammonia Process
In 1979, British Petroleum approached M.W. Kellogg to participate in
the development of a new ruthenium-based catalyst. It was known at
that time that replacing the conventional iron-based catalyst, which
was used for over 80 years, with this new catalyst would increase
productivity. Several pilot plant tests were performed using
ruthenium supported on a proprietary carbon structure with various
co-promoters.(6) It was evident that significant economic benefits
would be attained with this new catalyst, and so it was chosen to be
an integral part of what is now known as the Kellogg Advanced Ammonia
Process (KAAP).
KAAP can be implemented in one of three ways: as an expansion to an
already existing plant, as a retrofit design to an already existing
plant, or as a grassroots design when building a brand new plant. In
1988, KAAP had its first commercial exposure after Ocelot Ammonia
Company in Kitimat, British Colombia (now known as Pacific Ammonia
Incorporated or PAI), contracted M.W. Kellogg to evaluate its
existing ammonia plant for potential capacity increases and to
provide a suitable retrofit design. This company's ammonia plant was
ideal for KAAP's first demonstration since its synthesis loop had
separate feed streams for hydrogen and nitrogen and allowed the KAAP
reactor to be run under a variety of stream ratios.(7)
The KAAP system was successfully started up in November of 1992. It
consisted of a KAAP reactor which was installed downstream from the
magnetite converter already in existence. This KAAP reactor was a
two-bed radial flow converter with a unique proprietary sealing
system which avoided hot spots within the catalyst bed. The KAAP
catalyst was loaded in its oxidized state, just as most catalysts
are, although it is only active in the reduced state. Therefore,
fresh synthesis gas, which heated the catalyst bed to ~300o, was used
to for the reduction process. The synthesis loop operated at the
original design pressure of 2000 psia. Partially preheated feed exits
a 2-bed Kellogg converter loaded with magnetite catalyst and enters a
steam superheater and generator, which generates a pressure of 3000
psia. This feed stream, containing 15% ammonia, then passes into the
KAAP converter through a side inlet to the first bed. When the gas
leaves the second bed, ammonia concentration is increased to about
19%. A continuous purge is required in this retrofit because the
expanded plant contains more inerts than in the original synloop.
After the entire KAAP retrofit was completed, PAI had an energy
savings of 0.6 mmBTU/mt.(8) This more efficient and highly flexible
system has been very easy to operate and has paved the way for
grassroots facilities.
Grassroots designs are different from retrofit and expansion designs
in that they use 3 and 4 bed intercooled reactors. The first bed uses
conventional iron catalyst while the remaining beds utilize the
highly active KAAP catalyst. The reason for this is so that the iron
catalyst can take advantage of high ammonia reaction rates at low
ammonia concentrations. As the reaction progresses, however, the
ammonia concentrations increase, and the iron catalyst loses its
effectiveness.(9) The KAAP catalyst is then used to produce high exit
ammonia concentrations at low pressures, since it can be used at high
ammonia concentrations.
The grassroots ammonia plants typically utilize KAAP in conjunction
with KRES, the Kellogg Reforming Exchange System. Together, these
processes have a multitude of benefits, several of which stem from
the sole implementation of KAAP. The lower pressure synthesis loop,
which leads to significant capital savings, results from the use of a
single case gas compressor with thinner walled and lighter vessels,
fittings, and pipings. This synthesis loop is also advantageous in
that it uses energy more efficiently by recovering heat at a much
higher temperature, yielding a 40% decrease in energy conversion
relative to conventional designs.(10) Since the synthesis loop is
less complex than in other plants, operator attention is expected to
be less as well. In addition, all of these benefits bring with them
an expectation of greater reliability.
VI. Conclusion
The proposed ammonia synthesis design produces 1,016 metric tons/day
of ammonia at a feed of 5,500 kmol/hr. Although the lack of kinetic
data deterred the completion of the plug flow simulation, a Gibbs
reactor successfully emulated the desired results. The Haldor-Topsoe
plant in Clear Lake, Texas, was chosen as the model for the
conventional ammonia plant due to its comparable operation to the
proposed simulation and its use of iron-based catalyst. This process
could be further optimized by lowering compression costs and
utilizing a more efficient reactor. Replacing the conventional
catalyst with the new ruthenium-based catalyst in a multi-bed reactor
can achieve these goals. The industrial process may be safely and
productively operated at lower temperatures, thus reducing costs and
increasing profit.
M.W. Kellogg takes full advantage of this superior catalyst in its
breakthrough technology known as the Kellogg Advanced Ammonia
Process, or KAAP. KAAP, implemented as either a retrofit, expansion,
or grassroots design, has proven to have significant benefits, such
as reduced capital costs and energy savings. Kellogg's new ammonia
synthesis configuration leads to a economically advantageous and
flexible ammonia plant.
VII. Endnotes
1. Catalytic Ammonia Synthesis: Fundamentals and Practice, edited by
J.R. Jennings
Plenum Press, New York, NY, 1991.
2. Phone conversation with Haldor-Topsoe Ammonia Synthesis Plant in
Clear Lake, Texas.
3. Stephen A. Noe, "Catalytic Reactor Bed," US Patent 5 250 270
(1993) to Kellogg Company
4. Zbigniew Kowalczyk, Slawomir Jodiz, Jan Sentek, "Studies on
Kinetics of Ammonia Synthesis Over Ruthenium Catalyst Supported on
Active Carbon," Applied Catalysis A: General. vol 138 (1996)
p.83-91
5. Ibid
6. "KAAP: Kellogg Advanced Ammonia Process", The M.W. Kellogg
Company
7. Ibid
8. Ibid
9. T.A. Czuppon, S.A. Knez, R.B. Strait, "Commercial Review of KAAP
and KRES" The M.W. Kellogg Technology Co., presented at AIChE Safety
Symposium (Sept. 1996) Boston, MA.
10. J.R. Leblanc, "Ammonia 2000 Kellogg Technology for the Future",
Asia Nitrogen `96, Singapore
VIII. References
Catalytic Ammonia Synthesis: Fundamentals and Practice, edited by
J.R. Jennings
Plenum Press, New York, NY, 1991.
"Ammonia", Kirk-Othmer Encyclopedia of Chemical Engineering
Technology, 4th ed., Vol 2, (1991) pp. 638-691.
Anders Nielsen, Jorgen Kjaer. Bennie Hansen. "Rate Equation and
Mechanism of Ammonia Synthesis at Industrial Conditions", Journal of
Catalysis, Vol 3, (1964) pp. 68-79.
D.C. Dyson, J.M. Simon, "A Kinetic Expression with Diffusion
Correction for Ammonia Synthesis on Industrial Catalyst", I & EC
Fundamentals, Vol 7, (1968) pp. 604-610.
"Ammonia", Ullman's Encyclopedia of Industrial Chemistry, Vol A2,
(1980) pp. 152-209.
Zbigniew Kowalczyk, Slawomir Jodiz, Jan Sentek, "Studies on Kinetics
of Ammonia Synthesis Over Ruthenium Catalyst Supported on Active
Carbon", Applied Catalysis A: General, Vol 138, (1996) pp. 83-91.
Stephen A. Noe, "Catalytic Reactor Bed", US Patent 5 250 270 (1993)
to Kellogg Company.
"Ammonia", The M.W. Kellogg Technology Company.
J.R. Leblanc, "Ammonia 2000 Kellogg Technology for the Future", Asia
Nitrogen `96, Singapore.
T.A. Czuppon, S.A. Knez, R.B. Strait, "Commercial Review of KAAP and
KRES", The M.W. Kellogg Technology Co., presented at AIChE Safety
Symposium (Sept. 1996) Boston, MA.
"KAAP: Kellogg Advanced Ammonia Process", The M.W. Kellogg
Company.
IX. Appendix