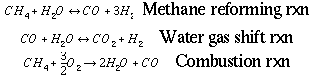
TABLE OF CONTENTS
ABSTRACT
TABLE OF CONTENTS
1.0 INTRODUCTION
2.0 OPTIMUM PROCESS
2.1 STEAM/CARBON SENSITIVITY
2.2 OXYGEN SENSITIVITY
2.3 PRESSURE SENSITIVITY
2.4 TEMPERATURE SENSITIVITY
3.0 REACTOR SIZING
4.0 PROCESS ECONOMICS
5.0 CONCLUSIONS AND RECOMMENDATIONS
REFERENCES
1.0 INTRODUCTION
Primary and secondary reformers play an important role in the
production of ammonia. The goal of reforming is to prepare as pure as
possible a gas mixture of nitrogen and hydrogen in a 3:1
stoichiometric ratio from the raw materials of water, air, and
natural gas. The reactions by which this ratio are achieved are given
as follows:
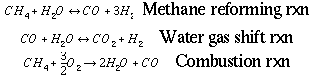
Reaction 1, the steam reforming reaction, and reaction 2, the
water gas shift reaction, are endothermic and occur in the primary
reformer. Reaction 3, the combustion reaction, is exothermic and
occurs along with reactions 1 and 2 in the secondary reformer [1].
Optimization of the reforming process involves the manipulation of
parameters to achieve high process yield while maintaining low
operating and installed costs. The parameters which are monitered in
this design include temperature, pressure, steam to carbon ratio, and
percent oxyegen in the air feed.
2.0 OPTIMUM PROCESS
The focus of this project is on the design of the primary and
secondary reformers. There are, however, several other reactors
necessary in any ammonia production process. These reactors can be
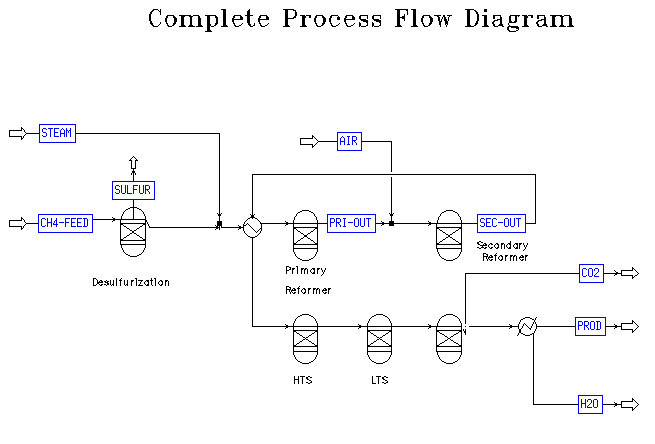
seen in the Process Flow Diagram (PFD) in Figure 1. A brief overview
of these other processes as well as the assumptions NGM Reformers
made in considering the effects of these processes on the primary and
secondary reformers will be discussed here.
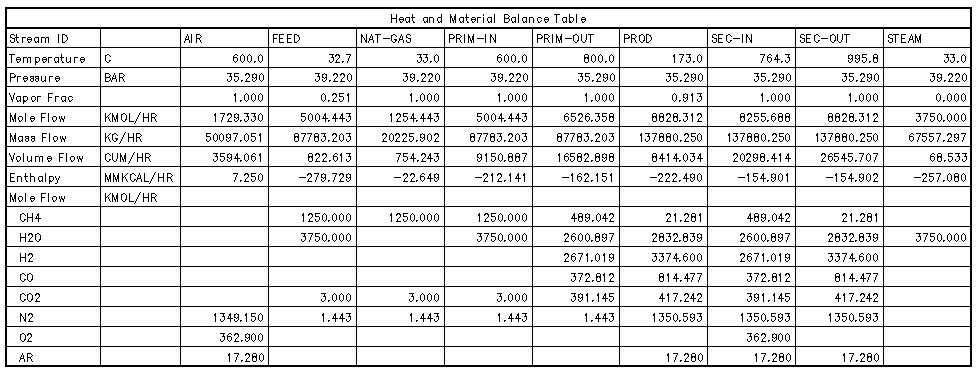
As seen in Figure 1, ammonia production begins with inlet streams of
natural gas and steam. For the optimum process, NGM chose a 20225.902
kg/hr natural gas feed stream composed of 99% methane entering at a
pressure of 35.29 bar. A 3:1 molar steam to carbon ratio was chosen
for the feed streams. The steam stream enters with a flowrate of
67557.297 kg/hr [2]. The methane feed is first passed through a
desulfurizer and then mixed with the steam feed. This mixed stream,
the primary reformer inlet stream of Table 1, next passes through a
heat exchanger where it is heated to a temperature of 600 C. From
here the primary reformer inlet stream enters the primary
reformer.
The primary reformer is modeled in ASPEN as an RGibbs reactor with RK
Soave equations of state. An operating temperature of 800 C and an
operating pressure of 35.29 bar are found to yield the optimum
process based on an analysis of sensitivity runs and industry
standards. The primary reformer is heat flux limited. Thus, based on
a calculated reformer heat duty of 50 MMkcal/hr, the reformer is
sized to contain 230 catalyst tubes with 4" inner diameter and a
length of 35'.
From the primary reformer, the primary outlet stream is mixed with a
600 C, 35.29 bar air stream. This mixed stream constitutes the
secondary inlet stream in Table 1.
The secondary reformer is also modeled in ASPEN as an RGibbs reactor
with RK Soave equations of state. An operating temperature of 996.2 C
and operating pressure of 35.29 bar are found to best optimize the
process. Based on these process conditions the secondary outlet
stream flows of hydrogen, carbon monoxide and nitrogen were found to
be 3375.48 kmol/hr, 814.378 kmol/hr, and 1350.593 kmol/hr
respectively. These numbers are critical to the production of 1000
metric tons per day of ammonia in the correct 3:1 stoichiometric
ratio. Based on the assumption that all downstream processes are
ideal, all the carbon monoxide found in the secondary reformer outlet
stream will be converted to hydrogen. Therefore, the addition of the
hydrogen and carbon monoxide should be in at least a 3:1 ratio with
nitrogen upon exiting the secondary reformer. This result is
accomplished and is evident in Table 1.
From the secondary reformer, the secondary effluent flows through a
heat exchanger where it heats the primary reformer inlet stream. From
the heat exchanger it passes through a high temperature shift
converter, low temperature shift converter, and methanator. For this
projec these processes were assumed to operate ideally at
equilibrium.
2.1 STEAM/ CARBON SENSITIVITY
Primary reformer inlet steam-to-carbon (s/c) ratio is an important
factor in reformer design. The literature advises the maintenance of
a relatively high s/c ratio to prevent mechanical as well as economic
problems during the life of the plant. Higher s/c ratios are more
effective for a number of reasons. First, because a high s/c ratio
favors the products in the reforming reaction equilibrium, it lowers
the amount of unreacted methane, or methane slip, out of the
secondary reformer and increases the production of hydrogen. Second,
a high s/c ratio inhibits the occurence of carbon-forming side
reactions in the primary reformer that result in carbon deposits on
the catalyst. Carbon deposition increases the resistance to gas flow
in the primary reformer tubes and may impair catalyst activity. This
impairment lowers the rate of the reforming reaction and can cause
local overheating or "hot bands" in reformer tubes that result in
premature tube wall failure. Finally, a high s/c ratio provides the
necessary steam for the shift conversion of carbon monoxide and
reduces the risk of carburization damage to the tube material
[3].
Table 2 shows process sensitivity to s/c ratio. A 3.0/1.0 s/c ratio
was found to be the most optimum ratio for the purposes of this
process. Sensitivity runs in Aspen showed that 4.0/1.0 s/c ratio
requires a larger heat duty than a 3.0/1.0 ratio. This increases cost
as more heat has to be applied to the process. However, lowering the
s/c ratio to 2.5/1.0 was found to increase methane slip
significantly, decreasing the amount of hydrogen produced. For these
reasons a 3.0/1.0 s/c ratio was chosen.
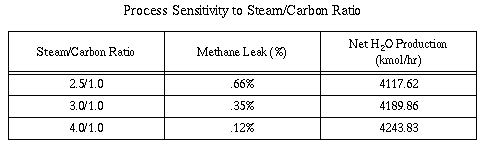
2.2 OXYGEN SENSITIVITY
Oxygen-enriched air is sometimes utilized in the production of
syn-gas as it shifts more of the reforming from the primary reformer
to the secondary reformer. An increase in the proportion of reforming
occurring in the secondary reformer results in a higher outlet
temperature from the secondary reformer. This heat can be recycled
and used to heat the primary reformer inlet stream to reduce energy
costs. On the other hand, enriched air introduces another cost to the
process by requiring that excess nitrogen be stripped from the
process downstream or that excess oxygen be purchased from a third
party supplier [4].
NGM Reformers Inc. decided that this extra cost exceeded the savings
gained from reducing energy costs. Hence, pure air (21% oxygen, 78%
nitrogen, 1% inerts) is used. Using enriched air also decreases
methane slip considerably, however, it decreases the production of
hydrogen to below product specifications. Table 3 shows process
sensitivity to the use of an enriched air stream.

2.3 PRESSURE SENSITIVITY
The chemistry, economics and demands from major clients must be taken
into consideration when analyzing the effects of reactor operating
pressures on the process. It is important to note that the process is
limited by a maximum pressure of 40 bar due to the metallurgy of the
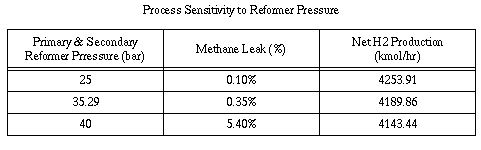
material used to construct the primary reformer tubes. Table 4
demonstrates the results of raising and lowering reformer pressure.
High reformer pressures near 40 bar favor the reactants of the
reforming reaction equilibrium, therefore, the production of hydrogen
decreases while methane slip increases. To compensate for high
methane slip the heat duty must be increased, thus increasing
compression and energy costs. Higher pressures also cause the
secondary reformer effluent temperature to decrease. This has an
unfavorable effect on the process as the heat from this stream is
used to heat the primary reformer inlet stream via heat exchanger. A
lower pressure of 25 bar exerts a favorable effect on the equilibrium
of the reforming process. According to the chemistry involved it
seems that lower pressures afford the most advantage; they increase
secondary reformer outlet temperature, decrease methane slip to about
0.01%, and increase hydrogen production by approximately 100
kmol/hr.
NGM Reformers, Inc. values customers in downstream ammonia synthesis
and realizes that their compression costs will be significantly
increased by low presures in the front end process. Hence, a process
pressure of 35.29 bar was adopted as a fair compromise, allowing for
the maintenance of a relatively low methane slip of 0.35% and a
sufficient throughput of product while maintaining a high enough
temperature in the secondary reformer outlet stream
2.4 TEMPERATURE SENSITIVITY
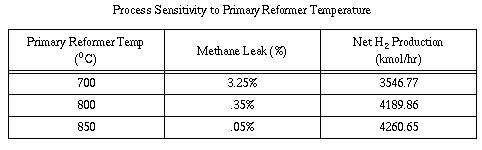
The reforming process favors high temperatures as it shifts the
reforming reaction equilibrium towards the production of hydrogen and
reduces methane slip. However, it is not advisable to operate the
primary reformer above 800 C because the metallurgy of the catalyst
tubes causes them to creep and bulge under the weight of the catalyst
at approximately 850 C. Additionally, the nickel catalyst melts at
1100 C. Operating at elevated temperatures also increases the heat
duty, causing energy costs as well as equipment costs to escalate
somewhat. In contrast, operating at 700 C decreases hydrogen
production and increases methane slip out of the secondary reformer
resulting in the waste of fuel. Table 5 shows these effects. The only
advantage of lowering temperature is a decrease in heat duty, which
will reduce costs somewhat.
Because of the large degree of process senstivity to primary reformer
temperature, it is desirable to operate at a temperature as close to
the metallurgical limit of 850 C as possible in order to maximize H2
production. Therefore a temperature of 800 C in the primary reformer
was chosen. This temperature is transferred to the adiabatic
secondary reformer. The secondary reformer is not constrained by
mechanical heat transfer surfaces; therefore, it can operate at
higher temperatures and is operated at 966 C outlet temperature.
3.0 REACTOR SIZING
Primary and secondary reformer reactor sizes were calculated from
industry data in order to minimize primary reformer size and thus
minimize installed cost. The primary reformer is heat flux limited;
that is, reactor size is determined based on the surface area over
which the necessary heat for reforming is transferred. A tube size of
4 inch I.D., 35 feet in length was chosen. This tube size is
consistent with industry averages [5]. The maximum conventional heat
flux through primary reformer tube walls is approximately 21,000
Btu/ft2*hr (5,921.176 kcal/ft2*hr) [6]. Using this value and the heat
duty through the reformer calculated by Aspen, the primary reformer
size was calculated as follows:
f = Maximum heat flux thorough tube walls = 5,921.176 kcal/ft2*hr
d = Heat duty through primary reformer (from Aspen) = 50.0771 x 106
kcal/hr
a = Total needed surface area of reformer tubes = d/f = 8457.28
ft2
t = a/36.7 ft2 per tube = 230 tubes needed
Catalyst volume was calculated from tube number and tube volume. The
primary reformer contains a total of 690 ft3 of catalyst.
The secondary reformer size was chosen based on industry input and a
length to diameter ratio of approximately one [5]. The reactor is 12
feet in diameter and 20 feet long. Ten feet of reactor length are
left void of catalyst so that combustion may occur away from the
catalyst.
4.0 PROCESS ECONOMICS
Approximate price ranges were obtained from M.W. Kellogg for the
primary and secondary reformers and catalyst. These prices reflect
the differences in construction materials used for each reactor. Due
to the high pressures and temperatures in the primary reformer tubes,
a 25% chromium-20% nickel alloy is the preferred tube material. The
secondary reformer, with its simpler design, can be priced as a
large, refractory-lined vessel containing a fixed-bed nickel catalyst
[7]. Primary reformers cost on the order of $5 million, secondary
reformers on the order of $1 million, and primary reformer catalyst
approximately $200/ft3 [5]. Therefore catalyst cost for the primary
reformer is $138,000, less than three percent of the total primary
reformer installed cost. Since the primary refomer is such a major
component of the process cost, the process was optimized so as to
minimize the size of the primary reformer. Less attention was given
to the amount of catalyst supplied to the primary reformer since it
becomes almost negligible when compared to the cost of the reactor
itself.
5.0 CONCLUSIONS AND RECOMMENDATIONS
In this process a 3:1 stoichiometric ratio of (H2 + CO) to N2 in the
secondary reformer effluent was achieved using a 90,000 kg/hr steam
plus natural gas basis feed with 3:1 steam to carbon ratio, 35.29 bar
reformer operating pressures, and 800 and 996 degrees C operating
temperatures in the primary and secondary reformers, respectively.
Several other factors may be considered, however, in designing a
fully optimum process for the required syngas output. These include
catalyst type, kinetic data, and reforming heat exchange.
The process modeled and optimized here is an equilibrium model. That
is, each reactor is assumed to be operating at equilibrium. In
reality however, equilibrium can not be reached, and an approach to
equilibrium model should be adopted. This involves the selection and
incorporation of the approprate kinetic data for the process. The
approach to equilibrium in the reformers is affected by choice of
catalyst. Numerous catalysts of differing properties are available to
suit specific purposes. Once a catalyst is chosen, parameters such as
catalyst activity, surface area, particle size, crush strength, and
nickel content among others should be considered to more accurately
model the process.
An alternative to the placement of the single heat exchanger used in
this process is to employ reforming heat exchange. This involves
recycling the secondary reformer effluent stream into the shell side
of the primary reformer vessel such that the heat exchange between
this stream and the primary reformer inlet occurs physically inside
the primary reformer vessel rather than through a seperate heat
exchange unit. This would require additional mechanical
considerations and an alteration of the construction of the primary
and secondary reforming vessels but is extremely thermodynamically
favorable. Reforming heat exchange may significantly reduce the size
of the primary reforming furnace and consequently decrease the cost
of the unit. Thus, reforming heat exchange should be considered as a
possible alternative heat recovery mechanism [5,7].
REFERENCES
[1]. Gerhartz, W. et. al. Ullmann's Encyclopedia of Industrial
Chemistry. 5th Edition. VCH, Federal Republic of Germany: 1985.
[2]. Mii, T. Process Systems Planning & Engineering Division,
Toyo Engineering Corporation, Japan. Electronic mail
correspondance.
[3]. Appl, M. Modern Production Technologies. British Sulphur
Publishing, London: 1997.
[4]. Ned, Zed, and Associates and The Autobots. "Design and Economic
Analysis of Ammonia Production Plant," CENG 404 Project Report,
Spring 1996.
[5]. Strait, R. B. Process Engineer, M.W. Kellogg Co., Houston, TX.
Personal interview.
[6]. Strelzoff, S. Technology and Manufacture of Ammonia. John Wiley
& Sons, New York: 1981
[7]. Kirk-Othmer. Encyclopedia of Chemical Engineering Technology.
4th Edition. John Wiley & Sons, New York: 1992.