Abstract
The major cost associated with industrial processes is the purchase of utilities needed to supply and remove heat from the units. The feedstock is a major cost, but is usually more than offset by the value of the product. Higher temperatures and pressures require that more energy be provided through heat transfer. By performing an analysis of all process streams, energy may be internally exchanged to avoid the unnecessary use of utilities. The capital cost of heat exchangers is far below the price associated with a constant utility demand over the plant lifetime. In our optimized design for the production of acetone from IPA, the utility costs outweigh the capital costs after only five years of operation; obviously, utility optimization is crucial in the process. By using the heat content of internal streams, the overall process is made more efficient and a higher profit may be achieved. We affected a net savings of $220,000 annually on utilities, which propagated through the process operating cost and created a 20 year net profit improvement of $10 billion. By running simulations on the entire process, slight modifications to unit set points may be studied to augment the heat integration with allowable process modification. Because two independent groups designed the separations and reactor units, the combined units had not been optimized together, and this presented the possibility that innovative changes were previously overlooked.
Introduction
We were selected to perform the optimization of heat exchange at an acetone production facility. Given the independent designs of the reaction and separations units, we were required to maintain product and wastewater purity specifications, while minimizing costs associated with the heat exchange in the process. Optimization was sought by evaluating possible exchanger networks, incorporating heat integration techniques outlined in the literature. Pinch analysis programs assisted the task by avoiding temperature crosses. The combined process was turned over to process simulators3 to complete the heat integration with process modifications.
A majority of the optimization resulted from heat integration techniques - the use of process energy to cool process streams with excess thermal energy by contacting them with energy deficient streams (i.e., cross a stream that must be cooled with a stream that must be heated). All process streams were investigated for their available utility in this process, including those streams within temperature specifications, to determine if by utilizing this energy, the process would remain within final purity specifications.
The conversion of isopropyl alcohol to acetone is endothermic, and therefore, heat must be provided for the reaction unit. The reaction occurs in the vapor phase requiring the isopropyl alcohol and water feeds to be vaporized by preheating. The reactor effluent stream must be cooled to 20° C to achieve sufficient removal of the hydrogen gas in the flash vessel. This extremely low temperature is needed to assure good separation of the hydrogen vapor from the acetone, which has a low vapor pressure at ambient temperature.
Since the reaction takes place at a high temperature (approximately 350° C), a molten salt is used to provide heat as the reaction consumes energy. Available thermal utilities included steam (high and low pressure), cooling water, and refrigerated water.
Discussion
Some major changes to the base case PFD were incorporated to utilize heat more effectively. In the base case, the reactor section includes an adiabatic reactor designed primarily to cool the product stream through added product conversion. Since this reactor absorbs much of the heat in the product stream, it removes the heat potential of this stream from future use in the overall process. Eliminating the second reactor by slightly increasing the size of the first reactor maintained product yield and purity while more efficiently using the heat in the reactor effluent.
Another departure from the base case involved the increasing the pressure in the flash tank. By increasing the pressure, we were able to increase the temperature of this vessel and decrease the vessel heat duty to zero. By analysis of the system sensitivity to changes in the temperature of this vessel, an optimum operating temperature to maintain product and waste stream specifications was attained while minimizing utility costs.
Our overall product purity was increased from the base case, and therefore, is still within plant specifications. Because information on product pricing is not readily available, no profit adjustments were performed to account for the higher purity product generated from our design.
The heat exchange system was analyzed using several methods. The calculations were initially done by hand on the base case using the methods described in reference 1 (pages 528-540). These calculations are included in the appendix. These calculations provided a first pass insight into the nature of the pinch point of this process and the minimum number of heat exchangers allowable. The results suggested the crossing of streams to utilize heat, but also showed the overall lack of process heat for use in integration. For this reason, we altered the design to allow more process heat to be used in heat exchange.
We also used the HYSYS modeling program to analyze the pinch points in our base case process. The results are listed in the appendix.
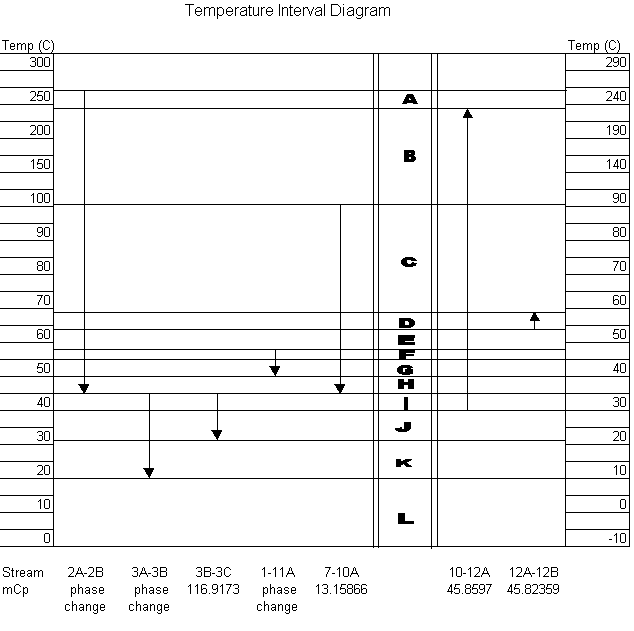
In order to improve upon the heat integration, a temperature interval diagram was constructed using a minimum approach temperature of 10 oC. The temperature interval diagram shown below includes all of the streams in the process that are either heated or cooled. A challenge to the heat integration arose from the number of phase changes in the process. This lead to difficulties with the calculations involved in the diagram, which was designed for same-phase streams only. Phase changes are normally handled by using dummy streams that have constant heat capacities. These dummy streams were found from the enthalpy vs. temperature graphs generated in HYSIS. No stream enthalpy (mCp) could be found from the information provided in HYSIS, so in the temperature interval diagram these streams were labeled "phase change."

Alternative uses of reactor heat were explored by comparing the use of the reactor effluent stream to preheat the feed stream. The main difference was the type of utilities needed to heat the feed and cool the product stream for introduction into the phase separator. If the product stream is used first to heat the feed stream, then the product stream can be cooled significantly and fewer utilities will be needed to chill stream 3 for the phase separator. This scenario mandates the use of high-pressure steam to complete the preheating of the feed stream. An alternate configuration would use low-pressure steam to heat the feed stream to the point at which subsequent contact with the product stream would result in full preheat. This configuration eliminates the costly high-pressure steam, but would require the use of much more cooling water to bring the product stream to the appropriate temperature for the phase separation step.
The ASPEN computer-modeling tool was used to simulate the entire process and to generate sensitivity data on temperature changes in the unit. By varying the inlet temperature to the phase separator, changes in the duties of the downstream column condensers and reboilers appeared. To determine the optimum operating range, the inlet temperature was varied and the temperatures and duties for the column heat exchangers were recorded. The product purity and final molar amount of product was also tabulated to ensure that production specifications were met. By assigning the appropriate flow to each of the exchanger utilities, each different temperature condition was assessed for viability; ultimately, the lowest cost configuration was selected. The best operating temperature for stream 3 (the phase separator inlet) is (preliminary investigation suggests ~35 C).
The final heat exchanger design resulted from the use of HYSYS and a 50% fouling factor to appropriately size the equipment. The fouling factor represents the amount of fouling that may occur while still allowing the required heat transfer. This reduces necessary down time for the cleaning of tubes, a costly and time consuming process. None of the process streams are corrosive or have the tendency to polymerize, so fouling should be a minimal concern.
Conclusions
The final optimized process is shown in the flow diagram below:
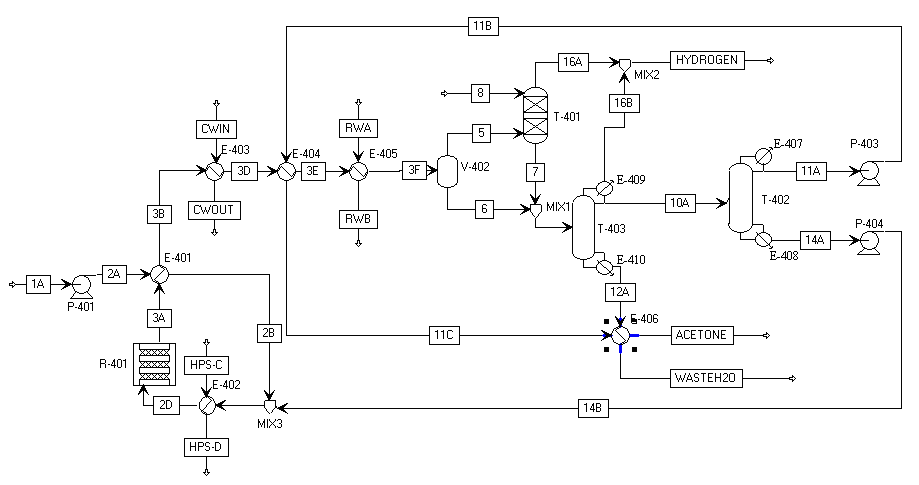
The most significant changes to the base case were the crossing of the reactor effluent with the inlet feed to the reactor. By eliminating the second reactor, we were able to maintain the temperature of the outlet stream at 350° C, which is enough to partially vaporize the feed. This reduces the steam needs for the unit, but we retain good reactor inlet temperature control through the high pressure steam unit located directly before the reactor (in case the effluent fails to completely heat the feed or if our molten salt stream fails). The final acetone product stream is also used as a cooling stream, due to its low temperature (~37° C). This reduces our refrigerated water duty from the inlet to the phase separator.
Cost Differences Compared to Base Case
|
COST |
OPTIMIZED |
BASE CASE |
|
|
Equipment |
(one time cost) |
$576,000 |
$366,000 |
|
Utilities |
Steam |
$192,896 |
$322,123 |
|
Cooling Water |
$5,722 |
$7,075 |
|
|
Chilled Water |
$701,760 |
$800,960 |
|
|
Fuel gas |
$54,400 |
$45,200 |
|
|
Waste Water |
$201,427 |
$205,296 |
|
|
Labor |
$700,000 |
$800,000 |
Exchanger Duty Chart
|
LPS |
HPS |
CoolingW |
RefrigW |
NatGas |
Electricity |
||
|
E-401 |
0 |
0 |
0 |
0 |
0 |
0 |
NO UTILS |
|
E-402 |
|
2.52 |
|
|
|
|
|
|
E-403 |
|
|
2.12 |
|
|
|
|
|
E-404 |
0 |
0 |
0 |
0 |
0 |
0 |
NO UTILS |
|
E-405 |
|
|
|
0.026 |
|
|
|
|
E-406 |
0 |
0 |
0 |
0 |
0 |
0 |
NO UTILS |
|
E-407 |
|
|
|
4.36 |
|
|
|
|
E-408 |
4.26 |
|
|
|
|
|
|
|
E-409 |
|
|
2.35 |
|
|
|
|
|
E-410 |
2.86 |
|
|
|
|
|
|
|
Furnace |
|
|
|
|
2.72 |
|
|
|
P-401 |
|
|
|
|
|
0.43 |
|
|
P-402 |
|
|
|
|
|
2.53 |
|
|
P-403 |
|
|
|
|
|
1.75 |
|
|
P-404 |
|
|
|
|
|
0.06 |
|
|
P-405 |
|
|
|
|
|
1.45 |
|
|
|
|
|
|
|
|
|
|
COST per utility |
$90,282 |
$102,614 |
$5,722 |
$701,760 |
$54,400 |
$2,986 |
|
|
TOT UTIL COST |
$957,763 |
|
|
|
|
|
|
Operating Expense Savings for Twenty Year Plant Life
$16,195,476 - $15,704,046 =$491K/year *20 years = $9.828 Billion
Due to our optimizations, the net savings over a twenty-year plant life are substantial. The refrigerated water and steam savings contributed to substantial utilities savings each year. The reduction in number of reactors also decreased labor needs. Also, a reduced wastewater duty and The detailed calculations for yearly operating cost are included in the appendix.
Recommendations
The product price probably varies appreciably with changes in product purity. Current analysis used initial design specifications of 99.9% purity by mole with a minimum production of 32.27 kmol/hr acetone product. Since this process produces pharmaceutical grade acetone, an investigation of the product pricing compared to purity should be made. This would allow further optimization of the unit to create the most profitable grade of acetone.
To find more precise sizing and cost data for heat exchangers, contact should be initiated with equipment vendors. Often these vendors have proprietary software that uses the input of exchanger data (stream compositions, flow-rates, and desired heat transfer rates) to produce specific recommendations on exchanger type and cost. The vendors also can determine appropriate fouling factors for each piece of equipment based on the process streams and coordinate the exchangers so they would all require cleaning at the same time, requiring only one shutdown to rework the exchangers, but getting the maximum lifetime between cleanings for all equipment.
Calculations
The heat content of each base case stream that is run through an exchanger appears in the following chart. These values include the latent heat involved when the stream undergoes a phase change.
|
Stream # |
2A |
3A |
3C |
11A |
14A |
10A |
12A |
12B |
|
Q (kW) |
59342.8 |
47300.4 |
-2922.9 |
18148.3 |
47.37 |
360.16 |
782.36 |
-2614.6 |
|
Total |
120444 |
Detailed Heat Exchanger Calculation
Per heuristics found in Turton, et al., U = 280 Watts/m2 ° C for liquid to liquid transfer. This is a conservative estimate for water service (water/liquid U values are listed as ~150, we choose to err on the side of safety). Using this U value, we will get a heat exchanger area that is large enough for the task at hand and also will probably allow some increase in flow later as the process is modified. A standard tube size is 1.9 cm OD.
Using these values, we calculate the required heat transfer area using the equation:
![]()
where q = heat transfer .8 = performance factor U = heat transfer coefficient
A = area of heat transfer D Tlm = log mean T difference across exchanger
F = correction factor for exchanger geometry (found using Y & Z values)
As an example, calculations of E-406 are shown. This exchanger is in service cooling the final wastewater stream before it is sent to the waste treatment facility. The stream is ~104° C and needs to be cooled to ~45° C. This achieved through a counter-current shell and tube heat exchanger. The cooling fluid is the final acetone stream as it exits the unit to product storage facilities. The acetone is heated in this process from ~37° C to ~56° C. This process requires ~45968 Watts to be transferred. Therefore, for a two shell pass and a multiple of four of tube passes:
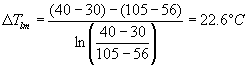
![]()
![]()
\ per chart, F = 0.9, this F is in agreement with Turton's heuristic value
![]() \
Area needed = 10 m2
\
Area needed = 10 m2
Since this estimate is quite conservative, the decision is made to reduce the area to the maximum allowed for a 1 foot diameter exchanger to save costs. So the area of design will be 9.3 m2. This required area would translate into a certain number of standard size tubes, with standard length of 4.9 m. The number of tubes is calculated by the following equations:
![]() \
Number tubes = 31.8 = 32 tubes
\
Number tubes = 31.8 = 32 tubes
References:
1. Analysis, Synthesis, and Design of Chemical Processes. Turton, Bailie, Whiting, and Shaeiwitz. Prentice Hall, New Jersey. 1998.
2. Matlab programs htxcc1, htxcc2 developed by Professor S.H. Davis, Rice University. These modules are particularly useful in calculating exchanger design data.
3. Engineering Heat Transfer. Welty, James R. Wiley & Sons, NY; 1974. pp. 17, 389-392.