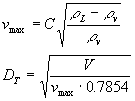The purpose of this project was to simulate the separation process that occurs during the production of 1-heptene and other unsaturated products from an initial mixture of C3 and C4 hydrocarbons. 1-heptene is the main product desired from this process since it has several applications including usage as a high-octane blending agent for gasoline and it aids in the production of plasticizers. The initial basis for our project was the process covered in the Turton text5 on pp. 736-746. The book describes the portion of the process that we analyzed as follows.
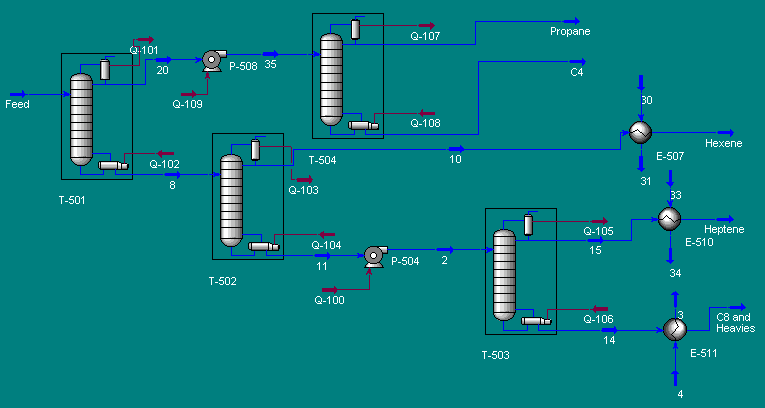
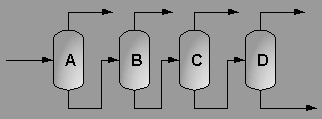
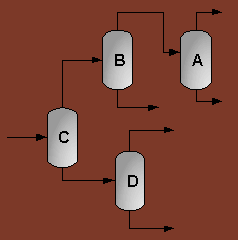
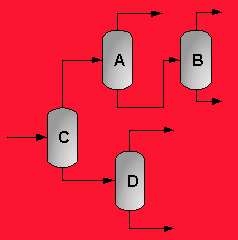
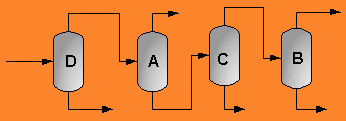
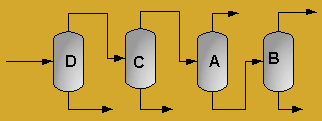
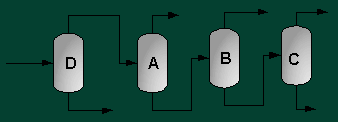
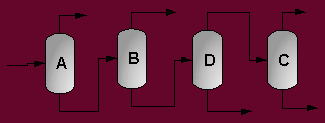
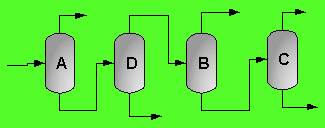
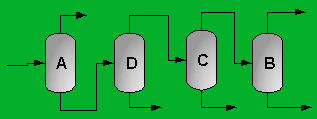
A partially vaporized reactor effluent is sent to the first of three columns. In the first column, the unreacted C3 and C4 hydrocarbons are separated from the rest of the materials. The C3 is then used as fuel gas while the C4 is sent to a LPG storage tank. The second column separates the 1-hexene from the remaining materials and sends it off as a product. Finally, the third column separates the desired 1-heptene product from the undesired C8 and heavier compounds. The C8 and heavier compounds are eventually processed off-site in order to remove the heavy materials and to hopefully recover some catalyst. The PFD given by Turton, et al. for the separation portion of the process is given below5.
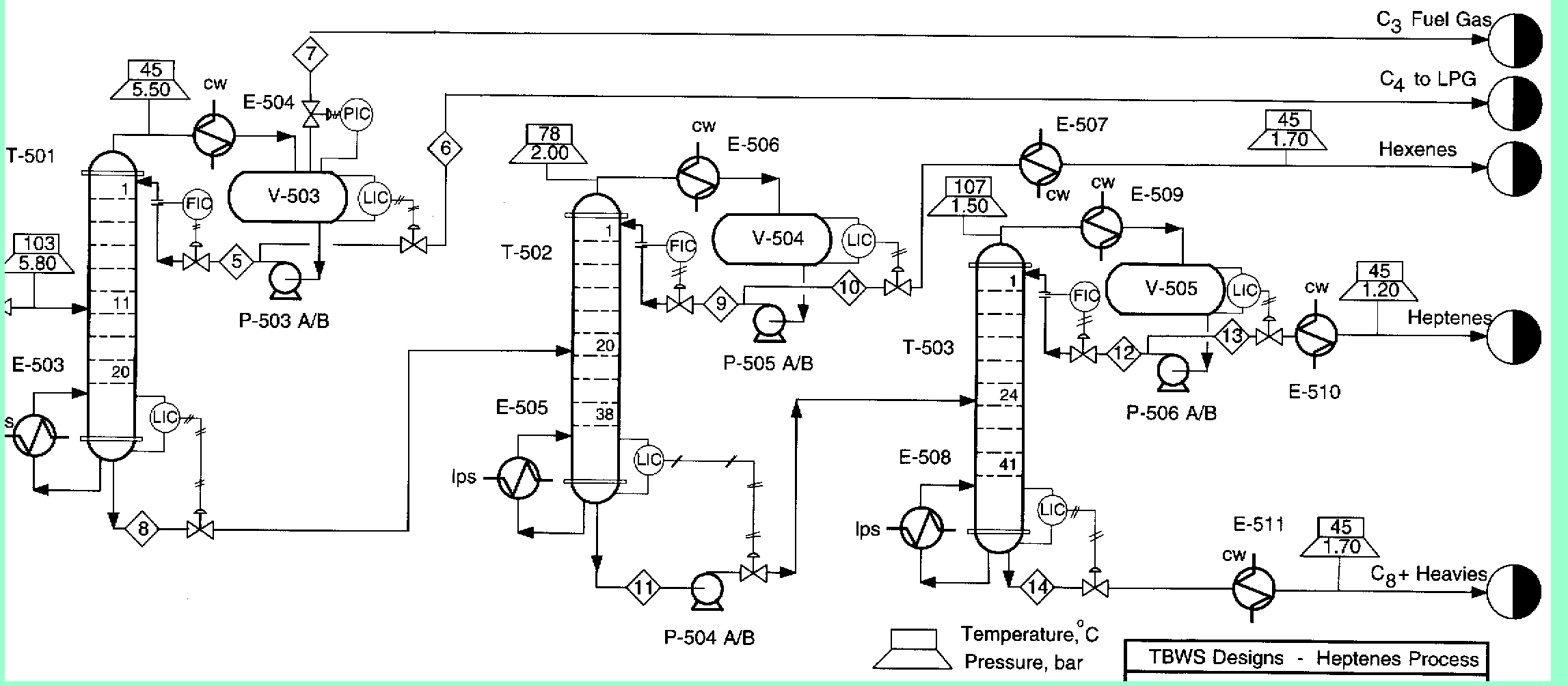
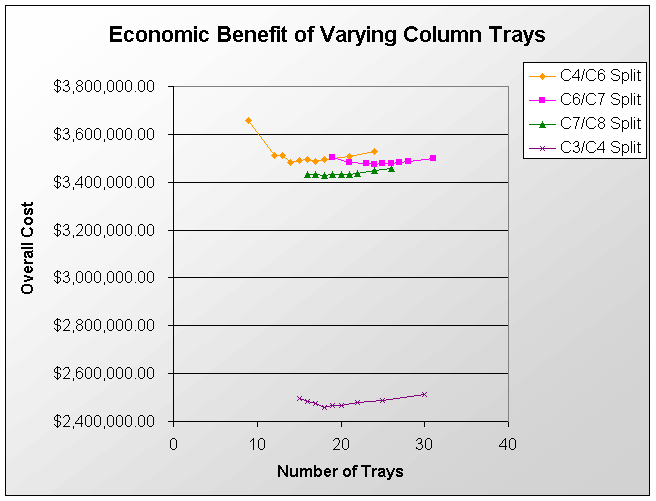
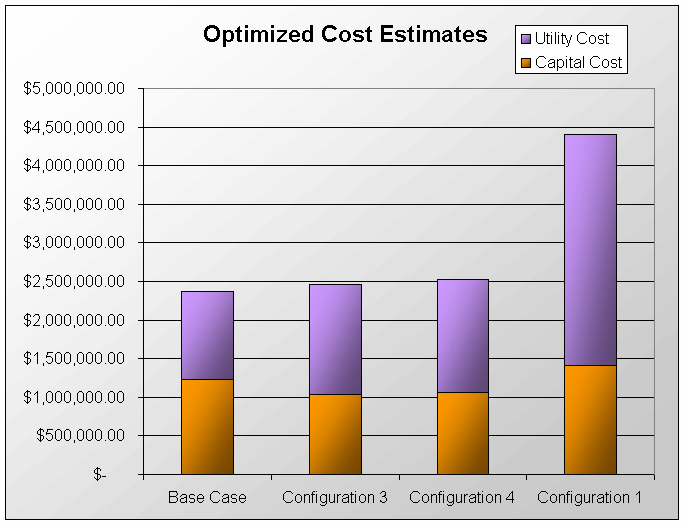
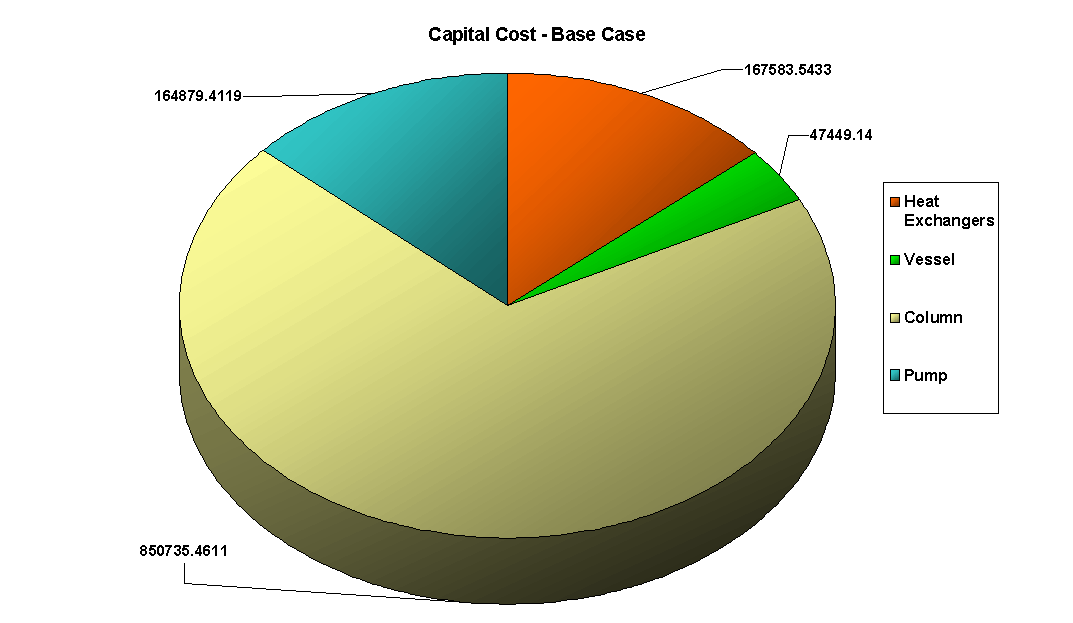
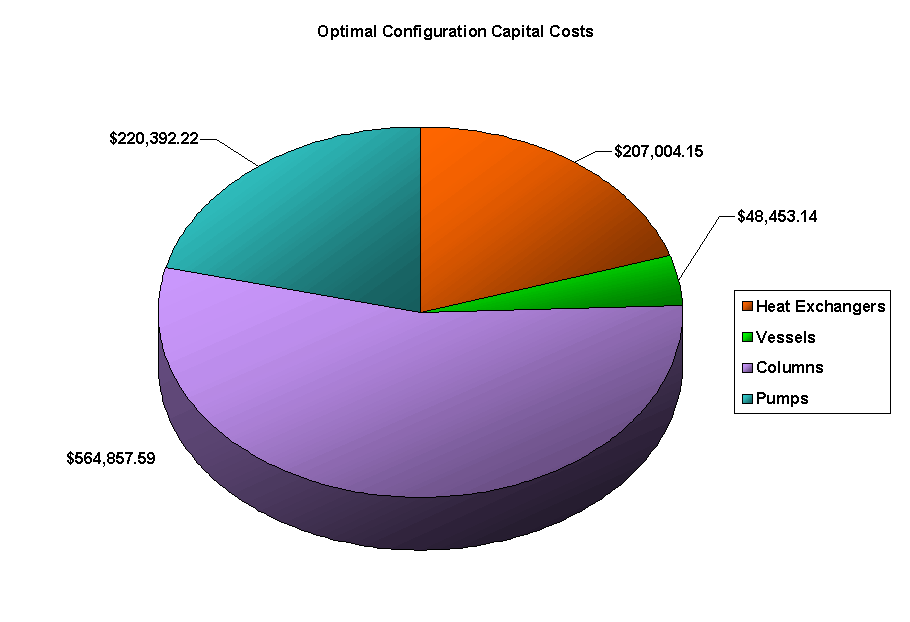
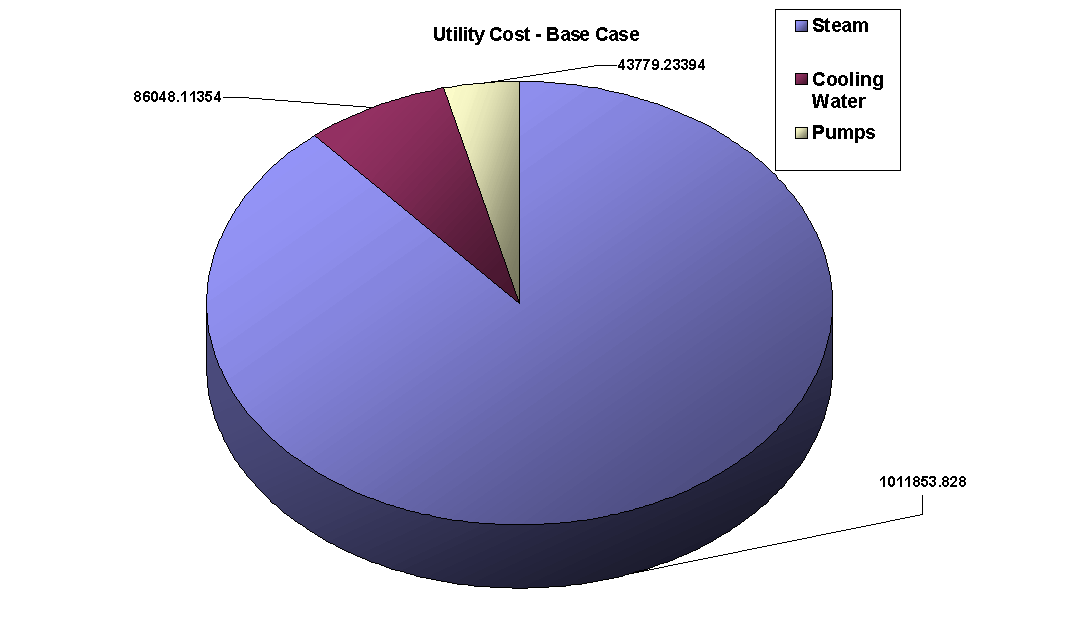
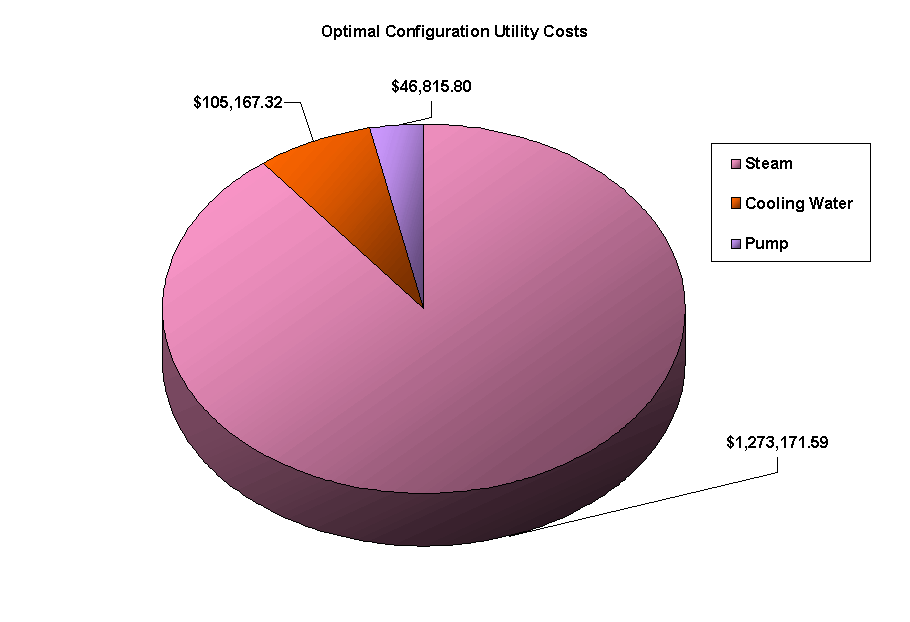
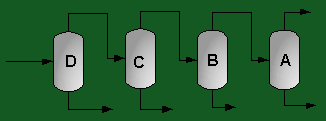
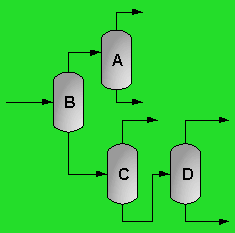
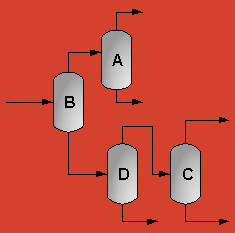
The above PFD is a preliminary estimate of the actual separation that occurs in the production of 1-heptene. It is then our job to improve this model by reducing cost and increasing profit without sacrificing the purity or output of the desired products. The rest of the report is dedicated to describing the methods we used to optimize the above process and to provide the corresponding results.
Design specifications that may not be altered due to feed stock supply and downstream equipment demand were agreed to as follows:
- The 1-Heptene stream should be 99% pure on a molar basis.
- No more than 0.2 kmole/hr of 1-Hexene may leave in the distillate of the first column.
- The 1-Hexene product stream should be 93% pure on a molar basis.
- The C3 fuel gas stream can contain moderate amounts of C4 impurity.