via the Catalytic Partial Oxidation of Propylene
Ceng 403 Project 1: Reactor Design
Group Seven



Kimberly Manney*
Gabriel Garcia
Brian Kirsch
Table of Contents
via the Catalytic Partial Oxidation of Propylene
Ceng 403 Project 1: Reactor Design
Group Seven



Kimberly Manney*
Gabriel Garcia
Brian Kirsch
Table of Contents
I. Abstract
II. Introduction
III. Base Case
IV. Catalysts
V. Reactors
VI. Safety
VII. End Uses
VIII. Proposed Design
X. Conclusions and Recommendations
XI. References
The production of polymers, VOCs and textiles is made possible through the common intermediate acrylic acid. The redefining of chemical plants to maximize yield of acrylic acid is an example of continuous engineering, and the market for acrylic acid is growing. Demand for better, more efficient, and cost effective processes is highly competitive. The following design took into account the different thermodynamic properties of the reactions and compounds to maximize a reactor, not only through cost efficient cut backs in cooling, but also through calibration of the reaction temperatures and yield. Although maintaining the reactor at a temperature of 190oC results in a yield of 94%, simulations showed that cooling the first 25% of the reactor to 190oC and allowing the rest of the reaction to equilibrate at 326oC will produce a yield of 96%. While simulating a reactor with a distinctive isothermal temperature gradient was easy, it is not feasible in real life. A two-reactor system is proposed as the realistic model. The first reactor will be 1 meter long, operate at 190oC, and will feed the 3 meter long second reactor operating at 326oC. This arrangement will also maintain side reaction yields at a minimum and hold oxygen, inert, and propylene under explosive limits. The steady state temperature of 326oC was determined by plotting a yield vs. temperature graph and visualizing the three steady states for inlet temperatures of 190-250oC. To resolve the new design, a steam-cooling stream is used to maintain the 1 meter long reactor at 190oC and a salt stream operates in case of emergency overheating for the 3 meter long reactor. Beyond all these alterations the total reactor length can also be decreased from the original 10m to 4 meters. It has been determined that the yield produced by the extra 6 meters does not justify the operating and capital costs. The total reactor length for the two reactors proposed by KGB Inc. is 4 meters. The final design has a lower heat duty of 58.7GJ/hr and a conversion of 96% in the isothermal case. The reduced heat duty, higher conversion, and lowering of exothermic side reaction yields justify the use of more complex design and two isothermal reactors in series.
This design project optimizes an acrylic acid plant. Acrylic acid is a valuable commodity chemical and is a beneficial chemical intermediate for many end uses. Traditionally, a two-reactor process converts propylene into acrolein in the first reactor, which is then converted to acrylic acid in the second reactor. Acrylic acid is formed by the following reaction:
C3H6 + 4.5 O2 à
3 CO2 + 3 H2O
Our design has modified the traditional two-reactor design. Rather than separating the reactors based on their products, we have "separated" them based on operating temperatures. KGB Inc. uses a two reactor system based on the operating temperatures of each reactor. The first reactor is 1 meter tall operating at 190oC. The second reactor, operates at the steady state temperature of 326oC. The features of KGB Inc.ís optimized Acrylic Acid reactor are as follows:
PFD 1 on the following page illustrates the base case that KGB Inc. optimized for the acrylic acid reactor. The specifications to meet were:
There are various catalysts that can be used for the single-step and two-step processes of producing acrylic acid from the oxidation of propylene. The catalysts involved in this process are complex oxides of molybdenum and vanadium of varying proportions. Several other metallic oxides can be used in lesser proportions in conjunction with molybdenum and vanadium. The catalyst can last for upwards of three years and still be economically effective.
We have chosen to use two reactors to convert propylene to acrylic acid. The reactor type is a shell-and-tube heat exchanger (plug-flow) reactor. The reactor consists of a cylindrical shell containing 1451 small diameter pipes. The feed stream flows into the small pipes, which are filled with catalyst. In the space outside of the pipes, a heat transfer medium flows co-currently with the product stream.
Our reactors will have some modifications made to it, however. The first reactor will have its temperature held at a constant 190 oC. The cooling fluid in the first portion will be steam contained solely in the first reactor. The temperature of the second reactor is at the steady state temperature of 326oC and will be cooled using molten salts.
The heat transfer medium for the first reactor was chosen to be steam. Due to the moderate operating temperature of the first stage, steam is the most viable alternative. Temperatures of less than 200 oC are not really suitable for molten salts, as they are still solids at those temperatures. Many plants use a molten salt stream for cooling purposes, and at the higher operating temperature of the second stage molten salt is very applicable. The higher temperature lends itself to molten salt due to its thermodynamic properties. KGB Inc. reactor specifications are as follows:
Acrylic acid is a dangerous and reactive chemical that must be treated with respect. Four main threats exist:
Polymerization can spontaneously occur with acrylic acid, releasing huge amounts of heat in an explosion. Consequently, inhibitors are used to stabilize the acrylic acid. However, these inhibitors only work in the presence of oxygen, so atmospheric air must exist in the storage container with the acrylic acid. Also, the acrylic acid must not be exposed to sunlight and should optimally be stored at temperatures between 15 and 30 degrees Celsius. Special care must be taken not to allow the acrylic acid to freeze, as it can polymerize upon thawing.
Production of large amounts of carbon dioxide from one of the side reactions can lead to dangerously high pressures, especially at very high temperatures. Our design produces a low yield of carbon dioxide. Finally, the last threat is exposure to the chemical itself. Its toxic effects can be avoided by using the appropriate protective equipment such as vapor proof goggles and masks.
Due to its reactivity, several materials are recommended for storage
of acrylic acid. These are stainless steel, glass, polyethylene or polypropylene.
For a large storage facility, stainless steel poses the best option in
terms of a reasonable building material.
Because Acrylic Acid can undergo the reactions of a carboxylic acid and also those of the double bond associated with acrylate esters, it is useful in polymer productions and also a common chemical intermediate. Acrylic Acidís primary use is in the production of acrylates. Polymers of acrylic acid and its salts are gaining in industrial significance. The central use for acrylic acid is in industrial coatings. Percentages of acrylic acid are copoly-merized with vinyl monomers for the preparation of water of solvent based coatings. Other uses for acrylic acid are as follows:
The background information and data for the design were calculated using ASPEN graphical simulation package. KGB Inc.ís proposed design can be seen in PFD 2 on the following page. The reactor and equipment specifications will be discussed in greater detail in the proposed design section of this report.
The results of the experiments would help in determining conditions necessary to maximize the process of acrylic acid production. The KGB Inc. designers tested the teamís criteria from last year and validated their results. Isothermal and adiabatic reactors were modeled at different operation limits and the results tabulated, graphed and compared with last years data. The following figure is a comparison between the KGB Inc. and 1998ís Group 4ís modeling data.
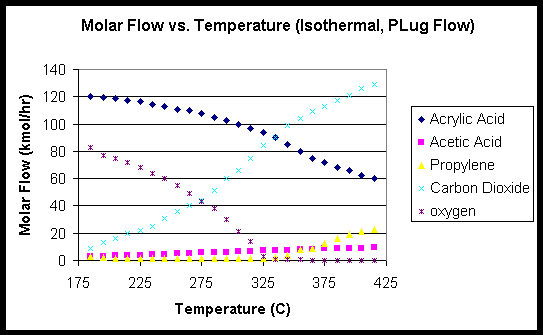 Figure 1
Figure 1
The isothermal data was obtained and graphed to validate last yearís case. The calculated yield for acrylic acid at 190oC is very high. Due to the nature of the reaction and the highly exothermic side reactions limited by the low temperatures, the reactor should operate at 190oC in a section long enough to yield a 70% conversion of propylene. Due to structural boundaries and heat transfer across mediums, operating one reactor at two different temperatures is not feasible. A two-reactor system will provide the desired yield while maximizing profits. To maximize profits the cost of cooling the first reactor to such a temperature must first be justified. Data was then collected from the adiabatic case with only the main reaction in a yield plug flow reactor. The outlet temperature for an inlet stream of 2342.2 kmol/hr was plotted against the conversion specified to provide an insight to the reaction kinetics. Figure 2 below has a unique property that is very important. The temperature of the outlet streams seems to plateau at 326oC and a 55-65% conversion of propylene. The fact that three steady states occur around the temperatures of 310-340 is very important in determining the inlet conditions and reaction safety.
Outlet temperatures as high as 1263íC result from a 95% yield. Thus operating at the higher steady state is not feasible. The middle steady state for an inlet temperature of 190oC is 326oC and 0.6 conversion of Propylene. Looking at Figure 1 the percent of product that is acrylic acid will decrease due to increased activity in the competing reactions. Running a plug flow reaction at inlet temperature of 190oC with an emergency cooling stream will result in a steady state temperature of 326 oC and a reasonable yield.
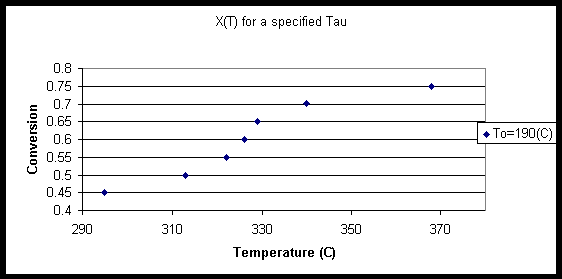
Figure 2
The benefits of this design is that a steady state has been reached and thus the temperature of the reaction does not fluctuate, reducing the utility costs considerably. It is important to reiterate that the reaction for Figure 2 does not consider the highly exothermic side reactions. To balance this out, a two-reactor system will minimize the side reactions by operating at different temperatures.
Once the inlet temperature, operating temperature, and expected yield for the isothermal cases were established, an adiabatic case was studied. Test runs with a molten salt coolant were created and executed to yield low flow rates of Acrylic acid, high concentrations of CO2 and very high temperatures. The following table illustrates these results.
Table 1
| Adiabatic Case, Plug Flow | ||
|
|
|
|
| Temperature (C) | 190 | 1732 |
| Pressure(bar) | 6 | 3.5 |
| Molar Flow (kmol/hr) | 2482.2 | 2503.4 |
| O2 | 280.9 | 0 |
| N2 | 1056.7 | 1056.7 |
| C3H6 | 127 | 37 |
| C2H4O2 | 0 | 5.3 |
| C3H4O2 | 0 | 142.3 |
| CO2 | 0 | 39.1 |
| H2O | 1017.6 | 1223 |
The runs of the adiabatic case were compared with an isothermal case in which the plug flow reactor was kept at the steady state temperature of 326oC. While the heat duty on this reactor was relatively low, the side reactions produced high pressures and flows of CO2. Table 2 shows these results, and also illustrates that the more CO2 produced the less acrylic acid produced.
Table 2
|
|
||
|
|
|
|
| Temperature (C) | 326 | 326 |
| Pressure(bar) | 6 | 5 |
| Molar Flow (kmol/hr) | 5472.314 | 5349.709 |
| O2 | 619.2785 | 93.81175 |
| N2 | 2329.625 | 2329.625 |
| C3H6 | 279.9871 | 0 |
| C2H4O2 | 0 | 53.32271 |
| C3H4O2 | 0 | 209.2772 |
| CO2 | 0 | 105.4855 |
| H2O | 2243.424 | 2558.187 |
The two cases prove that a combination of two reactors into one would probably result in a better selectivity for acrylic acid. An additional isothermal plug flow simulation, shown in Table 3, with a constant temperature of 190oC showed that acrylic acid selectivity is greatest at lower temperatures because the competing side reactions are minimized.
Table 3
|
|
||
|
|
|
|
| Temperature (C) | 190 | 190 |
| Pressure(bar) | 6 | 5 |
| Molar Flow (kmol/hr) | 5472.314 | 5343.601 |
| O2 | 619.2785 | 146.6749 |
| N2 | 2329.625 | 2329.625 |
| C3H6 | 279.9871 | 0 |
| C2H4O2 | 0 | 18.78239 |
| C3H4O2 | 0 | 249.9328 |
| CO2 | 0 | 52.61466 |
| H2O | 2243.424 | 2545.971 |
While the selectivity in this case is much greater, costs of cooling the reactor to 190oC are very high. The output flow rate of CO2 is also at low levels, producing a controllable pressure. KGB Inc. utilized these two ideas and modeled them into one. The most feasible design would consist of two reactors, one operating at a low temperature and the other at steady state temperature of 326oC. KGB Inc. designed a two-reactor system that:
Figure 3
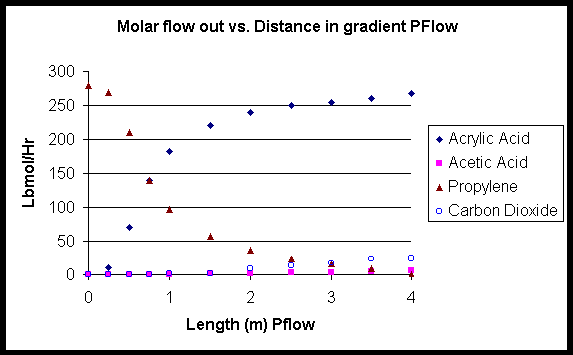
Figure 3 represents the output flow rates for different compounds at various distances down the reactors. The first meter is representative of the plug flow that is isothermal at 190oC thus limiting the side reactions that produce acetic acid and carbon dioxide. About 64% of the reactant propylene converts to acrylic acid in the unit length of the first reactor. Next, the output of the first reactor is fed into the second reactor with change conditions. The reaction is allowed to equilibrate at 326oC for the 3 meter second reactor. The reaction is not as selective to acrylic acid but because the concentration of O2 and propylene are so low there is very little heat produced from the exothermic reactions. An overall conversion of 96% is obtained for acrylic acid. The following table illustrates the output for variable temperature isothermal plug flow reactors of length 1 meter and 4 meters.
Table 4
|
|
|||
|
|
|
|
|
| Temperature (C) | 190 | 190 | 326 |
| Pressure(bar) | 6 | 5 | 5 |
| Molar Flow (kmol/hr) | 5472.314 | 5387.0548 | 5338.799 |
| O2 | 619.2785 | 434.0938 | 174.0828 |
| N2 | 2329.625 | 2329.625 | 2329.625 |
| C3H6 | 279.9871 | 96.9003 | 0 |
| C2H4O2 | 0 | 2.0031 | 5.78194 |
| C3H4O2 | 0 | 182.0674 | 267.7324 |
| CO2 | 0 | 1.1142 | 25.21009 |
| H2O | 2243.424 | 2341.251 | 2536.367 |
The side reactions are limited to only a small yield due to the low concentrations and optimal steady state conditions. Because the reaction is carried out at 326oC, the chance of an explosive reactor is easily controlled through a salt-cooling co-current stream that will maintain the reaction in the middle of three steady states. The fact that it is the middle steady state in itself gives the plant a buffer from which a control mechanism can effectively restore optimum operating conditions. The actual design of the reactor will have to depend on the preferences of the buyer. One 4 meter reactor can be made with a separation at one meter so that the cooling streams do not mix, or two reactors of respective lengths can be connected in series. The two scenarios have advantages and disadvantages. A two reactor system can be easily modified. A one reactor system may be more difficult to operate, but takes up less room and cost less to obtain. The cost analysis section will go further into the capital cost of the equipment.
Improving the design involved the fluctuation of different inlet stream concentrations, but due to the nature of the reactants very little was done to produce a greater yield. In the case of the adiabatic reactors, a decrease in oxygen flow provided a means of controlling the unusually high exit temperatures. Unfortunately this also led to the lowering of the acrylic acid yield from 96% to almost a third of the maximized case. Changes in reactor diameter and feed pressures seemed to be limited and in many cases highly costly. At this point the data does not justify the alteration of any of these properties to produce an overall profit. Keeping the reactor diameter at 3.6 meters allowed for enough room for the heat transfer tubing to enter without disturbing the output. Previous studies have determined that the last 5-6 meters in length of the reactor do not pay for themselves in product, thus the final sum of the reactors is only 4 meters long. By providing adequate cooling to keep the reaction at a temperature of 190oC for the first reactor and 326oC for the second reactor the final conversion of acrylic acid will be anywhere from 73% to 95.63%. If the operation is isothermal and steady state is achieved, a final conversion of 96% can be achieved resulting in 4,000 metric tons of acrylic acid being produced more than the base case of 50, 000 metric tons each year. The costs involved in having two heat exchangers and two pumps are well justified and constitute a minimal payback period.
Present refineries have incorporated a two-reactor system for many reasons. First, having two reactors will allow for expansion as well as dynamic system control. In reference to other industrial processes, the proposed design does not differ much in terms of hardware. Table 5 compares industry design to KGB Inc.ís proposed design and equipment requirements.
Table 5
| Sizes and typical operating conditions for major pieces of equipment in acrylic acid industries vs. KGB Inc. Design | |||||||
|
|
|
|
|
||||
|
|
Reactor 1 | Shell + tube type 5,000 to 10,000 tubes 0.75-2in I.d. * 10-20 ft long @310C | Shell + tube type 1,451 tubes 1 in I.d. * 3.34 ft long @190C | ||||
|
|
Reactor 2 | Shell + tube type 5,000 to 10,000 tubes 0.75-2in I.d. * 10-20 ft long | Shell + tube type 1,451 tubes 1 in I.d. * 10 ft long @326C | ||||
|
|
Compressor | Capacity 30,0000-50,000 ft3/min | Capacity 30,0000-50,000 ft3/min | ||||
|
|
Vaporizer | Q=1.5-2*10^6 Btu/h Shell Type 1000-2000ft^3 | Q=1.5-2*10^6 Btu/h Shell Type 1000-2000ft^3 | ||||
|
|
Steam pump | N/A | Q = 2-4*10^7 Btu/h 5000-7000 ft^3 | ||||
|
|
Salt Pump | Q = 2-4*10^7 Btu/h 5000-7000 ft^3 | N/A | ||||
|
|
Salt Pump | Q = 2-4*10^7 Btu/h 5000-7000 ft^3 | Q = 1-2.74*10^7 Btu/h 5000-7000 ft^3 | ||||
|
|
Bottoms cooler | Shell and tube heat exchanger Heat transfer are = 5,000-10,000 Ft^2 Q=3-5*10^6 Btu/h | Shell and tube heat exchanger Heat transfer are = 5,000-10,000 Ft^2 Q=3-5*10^6 Btu/h | ||||
|
|
Absorber | Absorber 10-15ft diam, 60-80 ft high | Absorber 10-15ft diam, 60-80 ft high | ||||
| Data: Encyclopedia of Chemical Processing and Design | |||||||
The reactors are built similarly except for the difference in total length. The additional length does not pay for itself in product. The new operating conditions are justified by the decrease in utility costs and increase in yield. Operation of the first reactor at 190 oC produces a stream that does not allow for side reactions to create a dangerous situation. The proposed design is both safer and economically beneficial. Operating at a semi-steady state yields the highest profits by cutting back on cooling costs.
The savings of KGB Inc.ís proposed design are seen in the revenue generated and the profit. Upon first glance, the costs of the raw material propylene, utilities, and capital costs are higher. Further investigations unveils that the cost of propylene is higher in 1999 taken from the Chemical Market Reporter than last year and the year the base case was designed. The following table compares the base case, the 1998 design by group four, and KGB Inc.ís costs, revenues, and profit.
Table 6
| Costs, Revenue, Profit | |||
| Base Case | 1998 | KGB Inc. | |
| Costs | |||
| Raw Material |
$11,948,000.00
|
$11,948,000.00
|
$13,208,832.00
|
| Utility (including labor) |
$2,400,000.00
|
$2,392,000.00
|
$2,168,808.00
|
| Capital Cost |
$2,478,000.00
|
$1,720,000.00
|
$1,509,000.00
|
| Total Cost |
$16,826,000.00
|
$16,060,000.00
|
$16,886,640.00
|
| Revenue | |||
| Acrylic Acid |
$79,490,000.00
|
$109,901,000.00
|
$104,777,719.20
|
| Acetic Acid |
$2,113,000.00
|
$1,051,000.00
|
$9,161,452.80
|
| Total Revenue |
$81,603,000.00
|
$110,952,000.00
|
$113,939,172.00
|
| Profit (1 year) |
$64,777,000.00
|
$94,892,000.00
|
$97,052,532.00
|
| Profit (5 years) |
$333,797,000.00
|
$481,340,000.00
|
$491,298,660.00
|
The total profit for the first year results in a ~50% savings over the base case and ~47% savings for a 5 year time span. The growth formula was used to calculate these savings.
Savings = [ final (KGB Inc.) Ė initial (base case) ] / initial (base case)
Further comparison of the base case costs and revenues vs. KGB Inc. are illustrated in Figure 4 below. The economics figures presented have an error of +/- 15%.
Figure 4
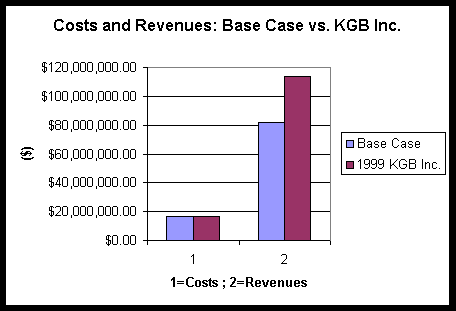
Conclusions and Recommendations
KGB Inc. proposed a distinctive design that is not only optimal but
also practical and profitable. Detailed study of the thermodynamics of
the acrylic acid production reactions have led to an optimization scheme
that is best for the process. By reacting 65% of the reactants in a vessel
at 190oC and then feeding the products into a second vessel
and allowing for semi-steady state equilibration at 326oC the
selectivity is increased, side reactions minimized, and utility costs reduced.
Profitable changes that do not minimize safety are as follows:
Armeniades, C.D. Personal Interviews.
Celanese Chemical Company. Product Description for Acrylic Acid. 1998
Celanese Chemical Company. Product Handling Guide for Acrylic Acid. 1998.
Encyclopedia of Chemical Processing and Design, J.J. McKetta, and W.A. Cunningham,
Ed. Vol 1, 404-413, 1976.
Kirk-Othmer Encyclopedia of Chemical Technology, 4th Edition, vol 1, 290-309,
John Wiley and Son, New York, 1991.
Turton, Richard et. al. Analysis, Synthesis, and Design of Chemical Processes.
New Jersey: Prentice Hall, 1998.
Ullmanís Encyclopedia of Industrial Chemistry, 5th Edition, vol A1, 161-171,
Verlagsgesellschaft (VCH), 1985.
United States Patent Office. Patent 3,825,600. Process for the Preparation of
Unsaturated Carbonyl Compounds.