Abstract
Abstract
MLA Process Works, Inc. has custom designed a separation unit for the Ruehl Corporation Ethanolamine Plant. The proposed design is an innovative approach to ethanolamine separation that optimizes costs, space, safety, production, and control. By implementing a single column with multiple product streams instead of a traditional series of three columns, the MLA design is capable of achieving high purity separation while reducing material and energy costs. Our separation unit produces 99.7% pure MEA, 97.5% pure DEA, and 99.6% pure TEA. The column is constructed of stainless steel and has an estimated cost of $445,200. Furthermore it is a versatile design that can be adapted to variable stream compositions and increased capacity. The MLA Process Works Separation Unit is our recommendation to the Ruehl Corporation as a look into the future of ethanolamine separation.
The Ruehl Corporation is interested in the production of monoethanolamines, diethanolamines, and triethanolamines. These products are commonly referred to as MEA, DEA, and TEA respectively. Ethanolamines are produced by the reaction of ethylene oxide with ammonia. They have a wide variety of uses ranging from cosmetics and pharmaceutical products to surfactants and gas sweeteners (McMillan). While market demand is high for all three products, it is most profitable for MEA. Since all three products are profitable though, the Ruehl Corporation would be benefited by achieving a high purity separation of all three products.
The Ruehll Corporationís current reactor process consists of two plug flow reactors. These reactors can be operated separately or in combination. This capability allows for two different production scenarios. In the first scenario, a single reactor is operated to produce a stream of predominately MEA. The second scenario uses two reactors in series, resulting in the production of a more even mixture of MEA, DEA, and TEA. This scenario optimizes DEA and TEA production. MLA Process Worksí proposed separation system of is capable of handling both of these production scenarios as well as any other variations in the feed stream.
In our endeavor to design an optimal ethanolamine separation unit, research was conducted on various methods and possibilities of separation. Due to the high boiling point of ethanolamines, liquid-liquid extraction was considered as an option in order to avoid high temperatures and heat costs. However the solubility characteristics of the different ethanolamines are too similar to make this and a feasible and advantageous process. Another option MLA considered is separation by crystallization. This method would take advantage of TEAí s relatively high solidification point. The high heat requirements, numerous disadvantages of solid transport over fluid transport, as well as the difficulties in obtaining a high purity separation economically discounted this as a possible method of separation. Thus, research indicated that conventional distillation would be the most plausible means of separation of ethanolamines.
Most older facilities employ a separation unit consisting of 4 to 6 distillation columns. This traditional design is used by the Huntsman Corporation as well as the Oxy Chem Petrochemicalsí Plant in Clear Lake, Texas. Oxy Chemís separation unit is composed of five columns: and ammonia column, a dehydration column to remove water in the product, and a series of columns separating MEA, DEA, high purity TEA, and low purity TEA. These last three columns are run at a vacuum to lower the boiling point of the ethanolamines. While this system works, it is not the most advantageous. MLAís separation proposal is a modern approach to ethanolamine production. This system minimizes cost and space, while at the same time enhancing production efficiency .
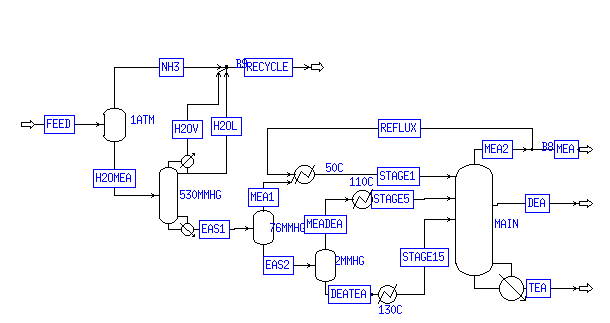
3.0 The MLA Process Works Ethanolamine Separation Unit:
Process Description
As previously mentioned, most ethanolamine separation units require four or five columns, each with 15 to 25 stages, to fully separate the reactor output into almost pure products. The MLA design accomplishes the same task with only two columns, without sacrificing energy efficiency or product purity.
The reactor can be run on a campaign basis, and according to the notes of Ruehl, et al, it can produce an output of either almost pure MEA, or a mixture of the three ethanolamines. The separation unit can handle either case, but because separating a multicomponent stream is more difficult, we used it as the design case. Ruehl did not list an accurate temperature for the overall reactor effluent, so we assumed the temperature would be slightly lower than that of the first ethanolamine reactor. The reactor feed is shown below.
Separation Unit Feed
|
MEA |
50.873 kmol/hr |
|
DEA |
19.497 kmol/hr |
|
TEA |
12.082 kmol/hr |
|
H2O |
91.283 kmol/hr |
|
NH3 |
287.472 kmol/hr |
|
Temp. |
160 ° C |
|
Press. |
68.046 atm |
Water, ammonia, and the amines all boil at very high temperatures at the high pressure of the feed stream. To separate the products without subjecting them to an overly high temperature, the pressure is dropped to atmospheric pressure in an unheated flash vessel. This low-cost, if primitive, separation method removes 96% of the ammonia and some of the water as vapor, which can be compressed and recycled to the reactors. The remaining water, ammonia, and amines are sent to the water separation column. A process flow diagram is presented in the appendix.
The water separation column contains 10 stages, and runs at a slight vacuum of 530 mmHg. The flash vessel liquid is fed at stage 3. A condensor, running with a reflux ratio of .3, keeps the top stage at 50 ° C, while a reboiler consumes 430 kcal/sec to keep the bottom stage at 176 ° C. All of the ammonia, and all but a trace of water, is separated into the column vapor stream. Between the flash vessel and the column, 3.3% of the fed monoethanolamine is also sent into the recycle. While in most plants this would be unacceptable, in this ethanolamine plant, it is desirable to return some of the MEA to the reactors. Since the plantís second reactor attempts to convert some of the MEA output into DEA and TEA; if additional MEA is recycled, it will help to supplant the efforts of this second reactor and may even make it redundant.
After removing reactants, the stream EAS1 (on PFD in appendix) carries 95 L/min of hot products to the ehtanolamine separation section of the unit. Because the ethanolamines must be separated under a vacuum, the stream experiences a second pressure drop, to 76 mmHg, in a second flash vessel. Half of the MEA fed evaporates, creating a vapor stream of 99.2% purity that can be sold to customers. By removing this product before the stream hits the column, the size of the column can be reduced, and its efficiency increased. The column feed contains almost equal amounts of MEA and DEA, along with a significant amount of TEA.
Most ethanolamine separation units use two or three columns in series, where MEA, then DEA, and finally TEA at high and low purity are removed. Our design takes advantage of a single 20-stage column, where the top half separates MEA from DEA, and the bottom half separates DEA from TEA. MEA is removed from the top of the column at 56 ° C, mixed with the vapor from the 76 mmHG flash, and refluxed as needed to keep the top of the column cool. DEA comes out of the center of the column, at stage 10, where the temperature is 123 ° C. TEA is removed from the reboiler, which provides 111 kcal/sec of heat to keep the temperature at 172 ° C.
To improve column efficiency, the column feed is split in a heated flash vessel. The vapor, largely MEA and DEA, is cooled to 110 ° C and sent to the top half of the column, at stage 5. The liquid, largely DEA and TEA, is heated to 130 ° C and sent to stage 15. These two feeds are important points of manipulation and control for the separation unit.
Overall, our separation unit produces 99.7% pure MEA, 97.5% pure DEA, and 99.6% pure TEA. Please see appendix for results.
There are several key advantages of implementing the MLA separation unit design rather than a conventional process. First, since only one column is needed in the ethanolamine separation section of the unit whereas formerly three were needed, facility space is greatly reduced. The MLA separation column is only slightly larger than one in a series in a conventional plant. Thus, material, heating, and piping costs are significantly lowered. Also, since TEA is unstable at high temperatures, the possibilities of it decomposing is minimized by exposing it to only one column and one reboiler. At the same time, the effectiveness of the column increases, since a very high quality separation can now be achieved by a single column as opposed to three separate ones.
Another advantage of this design is its ability to handle varying production levels. By adjusting the temperatures of the feed streams and the flow of the DEA stream, the column can be adapted to different feed compositions. If additional capacity is needed in the future, the stages can be replaced with commercial steel packing to significantly increase the columnís capacity.
Most equipment is standard and so only the major costs are presented in this report. For consistency, estimates were made in ASPEN although we have found costs at less than half of these figures at surplus equipment warehouses.
Assuming a tray efficiency of 53%, Aspen calculates the dehydration column to be 1.37m in diameter, and 7.6m in height. The cost is estimated at $76,000, assuming the column is built with stainless steel; a carbon-steel tower would cost $57,000. These costs exclude the condenser and reboiler price.
Assuming a tray efficiency of 65.3%, Aspen estimates the ethanolamine column to be 2.8 m in diameter and 19.2 m in height. The cost is estimated to at $246,000 using carbon-steel, or $445,200 if stainless steel is used. While carbon steel material is cheaper, we have found that ethanolamines react with the nickel component and can contaminate the products. Another option to stainless steel, is a stainless steel coating on the inside surface of the column. This method has been found to cause a variety of problems in industrial practice by many companies that we consulted, especially the Huntsman Corporation. Industry experts recommend a stainless steel column for the investment being made. So to maximize safety, durability, and the integrity of the final product, we recommend stainless steel columns throughout.
If natural gas is used for heat, the price of natural gas is considered to be $1.7/mmscf, and natural gas contains 1028 BTU/scf (figures as of 1997), the following costs will be incurred:
ASPEN Estimated Energy Costs
|
Item |
Energy Req. per Year (mm kcal) |
Cost per Year US Dollars |
|
H2O Column Reboiler |
13,347.72 |
$93,623 |
|
Main Column Reboiler |
3,452 |
$24,164 |
|
2 mmHg Flash Heater |
5,629.8 |
$39,409 |
|
DEA/TEA Stream Heater |
189 |
$1,323 |
There are other technically valid ways of carrying out this separation process. MLA Process Works, Inc. though is proposing is a top-of-the-line design. We are recommending to you an innovative separation unit that optimizes the process and the facilities. It is versatile and easily controlled while resulting in significant cost reductions in equipment, energy, and facility operation.
MLA Process Works, Inc. presents you with a modern design that is looking towards the future with less column maintenance, more column control, and a better separation at lower costs. We hope you find this proposal as exciting as we have.
Thank you for your time and consideration.
Sincerely,
C. Alejandra Garcia, Matt Reisdorf, Laurie Palombo
Project Leader Design Team Design Team
MLA Process Works, Inc. MLA Process Works, Inc. MLA Process Works, Inc.

ASPEN Simulation Report
STREAM SECTION
16 DEA DEATEA EAS FEED
----------------------
STREAM ID 16 DEA DEATEA EAS FEED
FROM : 76MMHG MAIN 2MMHG 530MMHG ----
TO : 2MMHG ---- 130C 76MMHG 1ATM
SUBSTREAM: MIXED
PHASE: LIQUID LIQUID LIQUID LIQUID LIQUID
COMPONENTS: KMOL/HR
MEA 22.8375 7.3046-03 0.3450 49.1683 50.8730
DEA 19.2984 19.3231 7.3442 19.4960 19.4970
TEA 12.0796 0.4695 11.3342 12.0819 12.0820
H2O 8.9955-05 3.0508-10 1.3926-07 1.4968-03 91.2830
EO 0.0 0.0 0.0 0.0 0.0
NH3 0.0 0.0 0.0 2.3823-11 287.4720
TOTAL FLOW:
KMOL/HR 54.2156 19.8000 19.0234 80.7479 461.2070
KG/HR 5226.1373 2102.0723 2484.1749 6855.6821 1.3500+04
CUM/HR 3.9787 1.6481 2.0659 5.6968 19.6138
STATE VARIABLES:
TEMP C 85.0000 123.1599 121.0000 176.7133 160.0000
PRES BAR 1.0000-02 2.7579-03 2.7579-03 0.7000 68.9480
VFRAC 0.0 0.0 0.0 0.0 0.0
LFRAC 1.0000 1.0000 1.0000 1.0000 1.0000
SFRAC 0.0 0.0 0.0 0.0 0.0
ENTHALPY:
KCAL/MOL -101.4783 -113.4305 -135.0282 -83.4814 -35.5809
KCAL/KG -1052.7309 -1068.4340 -1034.0266 -983.2653 -1215.5518
MMKCAL/HR -5.5018 -2.2459 -2.5687 -6.7410 -16.4104
ENTROPY:
CAL/MOL-K -161.3791 -172.3243 -206.4105 -131.6147 -49.4562
CAL/GM-K -1.6741 -1.6231 -1.5806 -1.5501 -1.6895
DENSITY:
KMOL/CUM 13.6264 12.0138 9.2082 14.1740 23.5143
KG/CUM 1313.5254 1275.4496 1202.4590 1203.4090 688.2969
AVG MW 96.3953 106.1652 130.5849 84.9022 29.2714
ASPEN PLUS VER: SOLARIS REL: 9.3-1 INST: RICEUNIV 10/25/97 PAGE 24
STREAM SECTION
H2OL H2OMEA H2OV MEA MEA1
-------------------------
STREAM ID H2OL H2OMEA H2OV MEA MEA1
FROM : 530MMHG 1ATM 530MMHG B8 76MMHG
TO : B9 530MMHG B9 ---- 50C
SUBSTREAM: MIXED
PHASE: LIQUID LIQUID VAPOR VAPOR VAPOR
COMPONENTS: KMOL/HR
MEA 0.9938 50.1627 5.4743-04 49.1611 26.3308
DEA 1.8405-05 19.4960 3.2003-11 0.1306 0.1976
TEA 4.3562-09 12.0819 9.9125-17 2.3872-07 2.3906-03
H2O 63.7295 65.7385 2.0074 1.4968-03 1.4069-03
EO 0.0 0.0 0.0 0.0 0.0
NH3 1.7059 11.4721 9.7661 2.3777-11 2.3777-11
TOTAL FLOW:
KMOL/HR 66.4293 158.9514 11.7741 49.2932 26.5322
KG/HR 1237.8666 8296.0700 202.5211 3016.7088 1629.5448
CUM/HR 1.2862 7.4141 449.8837 4.8995+05 7.8985+04
STATE VARIABLES:
TEMP C 50.0000 63.4178 50.0000 56.5765 85.0000
PRES BAR 0.7000 1.0000 0.7000 2.7579-03 1.0000-02
VFRAC 0.0 0.0 1.0000 1.0000 1.0000
LFRAC 1.0000 1.0000 0.0 0.0 0.0
SFRAC 0.0 0.0 0.0 0.0 0.0
ENTHALPY:
KCAL/MOL -66.4234 -75.3117 -18.7382 -48.8285 -48.4233
KCAL/KG -3564.5745 -1442.9609 -1089.4032 -797.8622 -788.4284
MMKCAL/HR -4.4125 -11.9710 -0.2206 -2.4069 -1.2848
ENTROPY:
CAL/MOL-K -38.4866 -92.6341 -19.1004 -69.1012 -70.0463
CAL/GM-K -2.0653 -1.7748 -1.1104 -1.1291 -1.1405
DENSITY:
KMOL/CUM 51.6461 21.4390 2.6172-02 1.0061-04 3.3591-04
KG/CUM 962.3922 1118.9556 0.4501 6.1572-03 2.0631-02
AVG MW 18.6343 52.1924 17.2005 61.1991 61.4175
ASPEN PLUS VER: SOLARIS REL: 9.3-1 INST: RICEUNIV 10/25/97 PAGE 25
STREAM SECTION
MEA2 MEADEA NH3 RECYCLE REFLUX
------------------------------
STREAM ID MEA2 MEADEA NH3 RECYCLE REFLUX
FROM : MAIN 2MMHG 1ATM B9 B8
TO : B8 110C B9 ---- 50C
SUBSTREAM: MIXED
PHASE: VAPOR VAPOR VAPOR MIXED VAPOR
COMPONENTS: KMOL/HR
MEA 61.4513 22.4925 0.7102 1.7046 12.2902
DEA 0.1633 11.9542 9.3168-04 9.5008-04 3.2667-02
TEA 2.9840-07 0.7453 5.2685-06 5.2728-06 5.9681-08
H2O 1.8710-03 8.9816-05 25.5444 91.2815 3.7421-04
EO 0.0 0.0 0.0 0.0 0.0
NH3 2.9721-11 0.0 275.9999 287.4720 5.9442-12
TOTAL FLOW:
KMOL/HR 61.6166 35.1922 302.2555 380.4590 12.3233
KG/HR 3770.8860 2741.9624 5204.1077 6644.4956 754.1772
CUM/HR 6.1243+05 4.1814+05 8411.7381 1.1942+04 1.2249+05
STATE VARIABLES:
TEMP C 56.5765 121.0000 63.4178 41.8244 56.5765
PRES BAR 2.7579-03 2.7579-03 1.0000 0.7000 2.7579-03
VFRAC 1.0000 1.0000 1.0000 0.8428 1.0000
LFRAC 0.0 0.0 0.0 0.1571 0.0
SFRAC 0.0 0.0 0.0 0.0 0.0
ENTHALPY:
KCAL/MOL -48.8285 -64.8172 -14.6871 -23.8458 -48.8285
KCAL/KG -797.8622 -831.9095 -853.0317 -1365.3955 -797.8622
MMKCAL/HR -3.0086 -2.2811 -4.4393 -9.0724 -0.6017
ENTROPY:
CAL/MOL-K -69.1012 -86.0599 -21.0105 -23.4068 -69.1012
CAL/GM-K -1.1291 -1.1045 -1.2203 -1.3402 -1.1291
DENSITY:
KMOL/CUM 1.0061-04 8.4164-05 3.5933-02 3.1858-02 1.0061-04
KG/CUM 6.1572-03 6.5575-03 0.6186 0.5563 6.1572-03
AVG MW 61.1991 77.9138 17.2175 17.4644 61.1991
ASPEN PLUS VER: SOLARIS REL: 9.3-1 INST: RICEUNIV 10/25/97 PAGE 26
STREAM SECTION
STAGE1 STAGE15 STAGE5 TEA
-------------------------
STREAM ID STAGE1 STAGE15 STAGE5 TEA
FROM : 50C 130C 110C MAIN
TO : MAIN MAIN MAIN ----
SUBSTREAM: MIXED
PHASE: LIQUID MIXED MIXED LIQUID
COMPONENTS: KMOL/HR
MEA 38.6210 0.3450 22.4925 2.7247-12
DEA 0.2303 7.3442 11.9542 4.2181-02
TEA 2.3907-03 11.3342 0.7453 11.6124
H2O 1.7811-03 1.3926-07 8.9816-05 3.6233-24
EO 0.0 0.0 0.0 0.0
NH3 2.9721-11 0.0 0.0 0.0
TOTAL FLOW:
KMOL/HR 38.8555 19.0234 35.1922 11.6546
KG/HR 2383.7222 2484.1749 2741.9624 1736.8999
CUM/HR 1.5967 6334.4634 3.7985+05 1.5613
STATE VARIABLES:
TEMP C 47.1410 130.0000 110.0000 173.1803
PRES BAR 2.7579-03 2.7579-03 2.7579-03 2.7579-03
VFRAC 0.0 2.7391-02 0.9345 0.0
LFRAC 1.0000 0.9726 6.5498-02 1.0000
SFRAC 0.0 0.0 0.0 0.0
ENTHALPY:
KCAL/MOL -63.9615 -133.9305 -66.4551 -147.3417
KCAL/KG -1042.5968 -1025.6199 -852.9312 -988.6679
MMKCAL/HR -2.4853 -2.5478 -2.3387 -1.7172
ENTROPY:
CAL/MOL-K -115.9409 -203.6523 -90.2818 -224.0490
CAL/GM-K -1.8898 -1.5595 -1.1587 -1.5033
DENSITY:
KMOL/CUM 24.3346 3.0032-03 9.2648-05 7.4643
KG/CUM 1492.8887 0.3921 7.2186-03 1112.4091
AVG MW 61.3482 130.5849 77.9138 149.0306