Chris Ruehl, Connie Hou,
Paul Lee, Lincoln Armstrong
CENG 403
Dr. Davis
October 7th, 1997
The objective of this project was to design the most efficient and most versitile system of reactors for a plant converting ammonia and ethylene oxide into ethanolamines. The three ethanolamines produced in this process are monoethanolamine (MEA), diethanolamine (DEA), and triethanolamine (TEA). Of these three products, MEA generally produces the highest profit margin; however, because all three products are valuable and are inevitably prduced in the reactors, and because in general the ethanolamine market is unpredictable and somewhat weak, any complete economic analysis of this plant will have to take all three products into account. The system proposed in this report makes use of an optional reactor and other adjustable process variables, such as the ammonia to ethylene oxide feedstock ratio, to increase the versatility of the plant and thus adjust the product distribution to fit whatever is most profitable at the present time. A final justification for increasing versatility at a higher cost of reactor installation is that the cost of the reactors in this system (estimated at $28,100 by ASPEN) is negligiable in comparison to the rest of the plant.
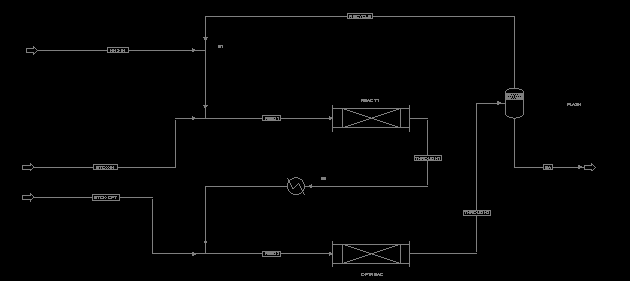
This system features two stainless steel, plug flow reactors, each
three meters long and thirty centimeters in diameter. The second
reactor is optional, and will only be used when a product
distribution roughy equal in the three products is desired. The inlet
stream to both reactors will be at 120 degrees C and 1000 psi, and
the temperature should not exceed 180 degrees C in the reactors.
Ethanolamines have found importance in the chemical industry as components of detergents, pharmaceuticals, and cosmetics. These chemicals are high-boiling, colorless, and viscous at room temperature. The wide-scale production of ethylene oxide (EO), the primary reactant, has made industrial ethanolamine synthesis possible. The combination of EO and ammonia produces MEA. However, since EO is incredibly reactive, the additional secondary products of DEA and TEA are produced. All three reactions are highly exothermic and can occur within a short reactor residence time. In industry these reactions occur in the liquid phase, and thus considerable compression costs are incurred in trying to maintain a liquid composition throughout the process.
Information about the specifics for the synthesis of ethanolamines is a hard sought commodity. Few quantitative, public studies have been done on ethanolamine synthesis. As a result, kinetic data for the reactions that produce ethanolamines are outdated and unreliable. In a 1966 study, Japanese researchers presented the following kinetic data:
|
k1=(4.1 + 4.0[H2O]2 x 102 x exp(-11,000/RT) |
|
k2=(7.2 - 0.042[H20])x k1 |
|
k3=(16 - 0.22[H2O]) x k1 |
in liter/mol min
where [H2O] is water concentration in mol/liter. However, when used in Aspen simulations, these data provided unreasonable results. Instead, kinetic data for Aspen simulations were obtained from a similar process simulation created by Professor Badgwell (see Bibliography). His data are as follows:
|
Z1= 9.302 x 10^8 |
E1= 7.085 x 10^4 |
|
Z2+ 2.464 x 10^9 |
E2= 6.899 x 10^4 |
|
Z3= 3.046 x 10^9 |
E3= 7.006 x 10^4 |
Exponential factors for the reactants for the plug flow Aspen model were assumed to be unity.
Additional process information was obtained through the help of
Nancy Dietrich (see Bibliography) at the OxyChem plant in Pasadena,
Texas. The OxyChem plant produces ethylene oxide derivatives on a
campaign, Ňas-neededÓ basis. The reactor section houses three
reactors that are capable of producing ethanolamines as well as
glycol ethers and acetates. This versatility enables the plant to
vary product outflow to follow market trends. Since the ethanolamine
market is in a slump and synthesis of MEA is the original intent of
design, additional consideration should be given in the production
and marketability of DEA and TEA. Therefore, the deciding factor in
the following design of the needed reactors is versatility in
producing different ratios of the ethanolamines.
There were several specific problems involved in the design of this reactor system. First, the solvent characteristics of the reaction needed to be determined. An anhydrous reaction would be simple and clean, but the reaction would have to occur in the liquid phase, and requiring compression of the ammonia to liquid phase would be a costly operation. The second option is to allow the reaction to occur in aqueous phase, thus allowing the ammonia to stay liquefied under lower pressures. This would also be a good method of reducing the reactivity of ethylene, for safety purposes (ethylene oxide can be explosive). The disadvantage is that the water would have to be separated later from the product stream, and also the fact that if anytime during the process the temperature or pressure of the process stream is too high, the ethylene oxide will react with the water to form ethylene glycol. It was decided to carry out the reaction in the aqueous phase because the safety gained with aqueous ethylene oxide and money saved by putting ammonia into solution would outweigh the cost of a separator and the glycol reaction would be minimal (rate of glycol formation doesnŐt begin to become near significant until about 430 C, and as will be discussed later the reaction never will reach temperature above 200 C).
Next we had to consider the temperature, feedrate, and reactor size and type for the reaction. Since the reaction is not auto-catalytic, we selected a plug flow reactor type to give the most efficient reaction for the space in the reactor. The reactor was made out of Stainless steel (SS316). It is important to note than one popular stainless steel, SS304, is made cheaply by substituting nickel and chromium for carbon. Nickel catalyzes a side reaction with ethylene oxide and MEA to form acid aldehyde, an impurity which also causes discoloration in the ethylene oxides and is a big problem if you sell TEA to cosmetics companies. Temperature is best around 120-160 degrees. If the reactor runs much hotter than that, the nickel-aldehyde reaction picks up in rate dramatically, and the product stream vaporizes, causing much difficulty downstream. Anywhere below around 120 C seriously hinders the rate of the reactions.
The reactions that take place are all exothermic and very fast. Thus, the reactor size does not need to be very big. In simulation, it was found that the theoretical reactor size was actually smaller than the feed pipe to the reactor, so the reactor was made to be a size accompanying the approximate diameter of the ammonia feed pipe plus a little extra to account for the amount injected of ethylene oxide in the reactor. The dimensions were 3 meters long and about 30 cm in diameter (internal volume .2120715 m3, residence time about 30 sec.). The extra length was to ensure that all of the ethylene oxide reacted. This was done on the suggestion of Nancy Dietrich of OxyChem, who informed us that any ethylene oxide left in the system after the reaction would react with something else downstream and increase corrosion and cause pressure differentials in the downstream processes that would be very damaging to that equipment. In general, the residence time of this reactor, based on a goal of 100 million pounds of ethanolamines per year, will be about thirty seconds. If only MEA is desired, the residence time can be reduced since MEA forms most quickly of all the potential products. This could also allow for more ammonia to flow through the reactor, increasing the ammonia:ethylene oxide ratio and thus the percentage of MEA formed.
Our design group decided to include an optional second reactor with a fresh ethylene oxide feed to improve on DEA and TEA production. The principal behind this is that the product stream from the first reactor being re-reacted with an equivalent amount of ethylene oxide would yield more DEA and TEA because the ratio of ammonia to ethylene oxide will be smaller (since some of the ammonia was consumed in the first reactor) and that the feed to the optional reactor now would now contain MEA, which can be reacted directly to DEA and TEA. This option worked extremely well, converting the stream of mostly MEA from the first reactor to get about 2 parts MEA to one part DEA to 2/3 part TEA. in the second reactor output.
After the second reaction, the heat generated is partially consumed by the ammonia as it comes out of solution. The feed stream is then fed to a flash tank which allows the gaseous ammonia to vent out the top and be recycled, while the remaining liquid (containing the products) is taken off for further separation. If the optional reactor is not used, the product can be fed straight to the flash tank by adjusting two valves. The first product stream is reliqified by a cooler in between the first and second reactors if the optional reactor is to be used.
The total capital cost of this reactor system is $28,100 (see
ASPEN economic analysis, Appendix E). This cost is obviously
negligible in comparison to the cost of the plant, another reason to
increase the versatility of the reactor system. The specific
breakdowns of this cost can be found in Appendix E. Although prices
in the ethanolamine market fluctuate, in general the cost of
ethanolamines is not much, if at all, higher than ethylene oxide. As
such, the major profit from this plant will be realized through the
conversion of ammonia to ethanolamines. This fact should be kept in
mind when the operating conditions are determined (see the next
section).
This reactor system can be run at a variety of operating conditions, depending on the desired product distribution and other economic factors (i.e. price of reactants). As such, there are a number of different variables in the operating conditions that can be adjusted to get the desired products and minimize production costs. The first consideration is to determine the optimum ratio of NH3 and ethylene oxide (EO). High ratios of NH3 to EO lead to high ratios of MEA in the product stream (see graph in Appendix), which is desirable because it is generally the most profitable of the three products. However, high NH3 levels mean a larger recycle stream, which increase compression costs and separation costs of water. The ASPEN model of this system indicates that a 10:1 ratio of NH3 / EO was sufficient to produce virtually all MEA, and a that roughly a 3:1 ratio can lead to a more even distribution of the products. Appendices C and D are the results from two ASPEN models of our system, geared towards high-MEA and even product distribution, respectively. Note that in the first case only the first reactor is used, while in the second case both are operating.
As mentioned before, the reaction is carried out in the aqueous phase for safety reasons, but the water is also necessary to make the reaction proceed. Ideally, the water content of the process stream should be minimized to minimize separation costs. The ASPEN model of the system featured roughly 90% NH3 in H20 by mass in the feed stream.
The design incorporates two of these reactors to increase the
versatility in terms of the product distribution. The first reactor
produces virtually all MEA, and since most of the time MEA is the
most desirable product, the second reactor is optional. However,
since one cannot accurately forecast the demand for DEA and TEA, the
second reactor is available to increase the yield of these products
as desired. The price of the reactors is very minimal compared to the
compression and heating costs in the other parts of the plant, thus a
small investment in a second reactor at the present time would be a
prudent safeguard against fluctuations in the ethanolamines
market.
Badgwell, Dr. Thomas A., contact
Dietrich, Nancy, contact, (281)474-0726
H. Hammer, "Ethanolamines and Propanolamines," in UllmanŐs Encyclopedia of Industrial Chemistry, W. Gerhartz, ed., VCH Verlagsgesellschaft, mbH, Cambridge, New York (1987), pp. 1-22.
M. Hatta, T. Ito, M. Miki, T. Okabe, "Reaction of Ethylene Oxide with Ammonia," Yukagaku 15 (1966) no. 5 215-220.
T. McMillan, "Ethylene Oxide Derivatives," SRI
International 193 (1991) 6:1-46.

=============================================================
Data file created by ASPEN PLUS Rel. 9.3-1 on 15:44:27 Sun Oct 5, 1997
Run ID: RES1 Item: STREAM-SUM Screen: Stream-Sum.Main
C-----------C-----------C----------C----------C----------C----------C-----------
Display ALLSTREAMS 1FEED 2FEED EFFL1 EFFL2
Units: METCBAR From: B1 OPTMIX REACT1 OPTREAC
Format: GEN_M To: REACT1 OPTREAC COOL FLASH
Phase: MIXED LIQUID MIXED LIQUID
Temperature [C] 125.0 125.0 178.7 125.0
Pressure [BAR] 68.948 68.948 68.948 68.948
Vapor Frac 0.024 0.000 0.625 0.000
Mole Flow [KMOL/HR] 1033.369 931.051 931.050 931.051
Mass Flow [KG/HR] 20500.000 20500.045 20500.000 20500.045
Volume Flow [CUM/HR] 47.381 32.403 261.462 32.403
Enthalpy [MMKCAL/H -21.882 -25.038 -21.882 -25.038
Mass Flow [KG/HR]
NH3 13500.000 11800.597 11800.597 11800.597
EO 4507.455 0.057 0.013 0.057
MEA 5940.776 5940.776 5940.776
DEA 265.549 265.549 265.549
TEA 0.521 0.521 0.521
H2O 2492.545 2492.545 2492.545 2492.545
Mass Frac
NH3 0.659 0.576 0.576 0.576
EO 0.220 3 PPM 616 PPB 3 PPM
MEA 0.290 0.290 0.290
DEA 0.013 0.013 0.013
TEA 25 PPM 25 PPM 25 PPM
H2O 0.122 0.122 0.122 0.122
Mole Flow [KMOL/HR]
NH3 792.693 692.907 692.907 692.907
EO 102.319 0.001 <0.001 0.001
MEA 97.256 97.256 97.256
DEA 2.526 2.526 2.526
TEA 0.003 0.003 0.003
H2O 138.357 138.357 138.357 138.357
Mole Frac
NH3 0.767 0.744 0.744 0.744
EO 0.099 1 PPM 308 PPB 1 PPM
MEA 0.104 0.104 0.104
DEA 0.003 0.003 0.003
TEA 4 PPM 4 PPM 4 PPM
H2O 0.134 0.149 0.149 0.149
=============================================================
Data file created by ASPEN PLUS Rel. 9.3-1 on 17:14:43 Sun Oct 5, 1997
Run ID: RES2 Item: STREAM-SUM Screen: Stream-Sum.Main
C-----------C-----------C----------C----------C----------C----------C-----------
Display ALLSTREAMS 1FEED 2FEED EFFL1 EFFL2
Units: From: B1 COOL REACT1 OPTREACT
Format: GEN_M To: REACT1 B4 OPTMIX FLASH
Phase: MIXED LIQUID MIXED LIQUID
Temperature [C] 125.0 125.0 244.7 125.0**
Pressure [BAR] 68.948 68.948 68.948 68.948
Vapor Frac 0.024 0.000 0.866 0.000
Mole Flow [KMOL/HR] 490.648 509.540 412.873 461.206
Mass Flow [KG/HR] 10500.000 13500.119 10500.119 13500.119
Volume Flow [CUM/HR] 23.787 18.995 186.431 18.192
Enthalpy [MMKCAL/H -9.170 -15.598 -9.170 -16.941
Mass Flow [KG/HR]
NH3 6300.000 5100.046 5100.046 4895.806
EO 3426.255 2129.254
MEA 3862.243 3862.243 3107.493
DEA 750.784 750.784 2049.854
TEA 13.301 13.301 1802.475
H2O 773.745 1644.490 773.745 1644.490
Mass Frac
NH3 0.600 0.378 0.486 0.363
EO 0.326 0.158
MEA 0.286 0.368 0.230
DEA 0.056 0.072 0.152
TEA 985 PPM 0.001 0.134
H2O 0.074 0.122 0.074 0.122
Mole Flow [KMOL/HR]
NH3 369.923 299.464 299.464 287.472
EO 77.775 48.334
MEA 63.229 63.229 50.873
DEA 7.141 7.141 19.497
TEA 0.089 0.089 12.082
H2O 42.949 91.283 42.949 91.283
Mole Frac
NH3 0.754 0.588 0.725 0.623
EO 0.159 0.095
MEA 0.124 0.153 0.110
DEA 0.014 0.017 0.042
TEA 175 PPM 216 PPM 0.026
H2O 0.088 0.179 0.104 0.198
** The optional reactor was modeled as a RSTOIC reactor in ASPEN.
Conversions were estimated based on literature data corresponding to
the NH3 / EO ratio used. As such, temperature and pressure outlets of
this reactor are innaccurate.
=============================================================
Data file created by ASPEN PLUS Rel. 9.3-1 on 17:18:55 Mon Oct 6, 1997
Run ID: RES1 Item: CS-1 Screen: Cost-Section.Direct-Cost
C-----------C-----------C----------C----------C----------C----------C-----------
Description: Units:
MATR-COST LABR-COST MAT & LAB LABR-HOUR
Equipment $ 11700 11700
Equip Set'g $ 800 800
Piping $ 4900 2500 7400 100
Concrete $ 700 600 1400 0
Steel $ 0 0 0 0
Instrumentat$ 500 100 600 0
Electrical $ 400 300 700 0
Insulation $ 500 400 1000 0
Paint $ 100 200 300 0
Misc $ 0 0 0 0
Tot Commd'y $ 7200 4200 11400 200
Building $ 2600 1400 4000 100
Testing $ 0 0 0 0
Additional $ 0 0 0 0
Spare $ 300 300
Total Other $ 2800 1400 4300 100
Total Unit $ 21800 6400 28100 300