Two examples will be shown in this section of the notes and compared with results from Matlab and results reported in Thermodynamics texts:
The Matlab simulations may be found in:
The summary comparison among the three procedures may be found at:
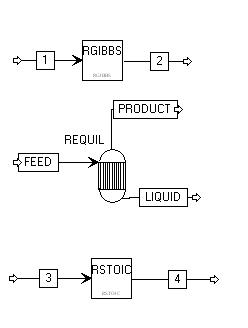
An equilibrium reactor may be simulated by using either the REQUIL or the RGIBBS reactor modules in Aspen. We will demonstrate both. Here is the flow diagram that was used in all of the simulations:

Here are the compounds:
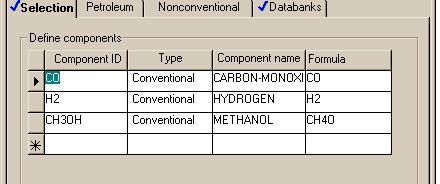
Stream 1 and the FEED stream were set with the same flows as we will see in the output from these runs. The conditions in the reactors were also set to be identical for each of the following cases:
For example the first two cases were set with the properties:
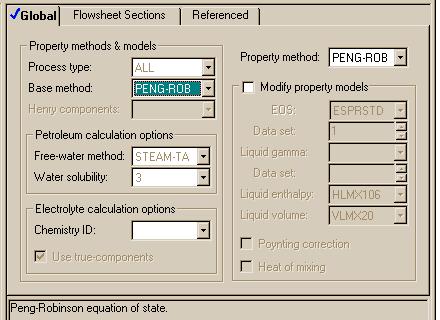
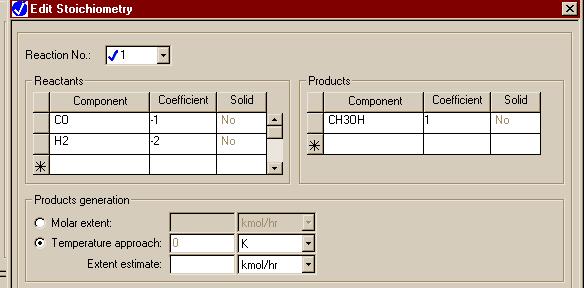
Note that for the REQUIL and RSTOIC modules, we must fill out forms that give the stoichiometry of the reaction. Here is the one set for our reaction in the REQUIL module:
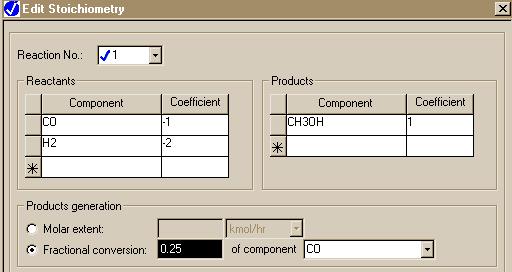
Here is the similar form for the RSTOIC module:
You must remember several things about these forms:
Here are the results for this first case for the inlet and exit streams to REQUIL and RGIBBS:
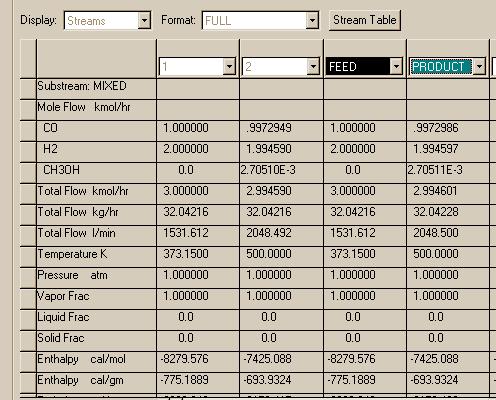
The REQUIL and RGIBBS reactor modules produce the same results in this and agree with what was found using the Matlab programs. Now let's try the high pressure case:
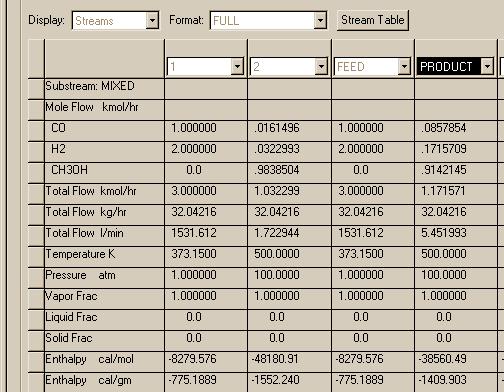
Here we find that the RGIBBS reactor module predicts much higher conversion than the REQUIL module. If we search for other checks on this problem, we find that the use of the Peng-Robinson thermo package may produce errorneous results at high pressures for compounds such as hydrogen. Let's try another package called PR-BM. It is a modified form of the Peng-Robinson package and should be more reliable:
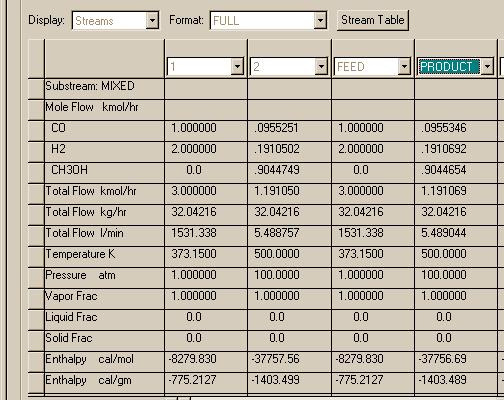
A much better agreement. Closer inspection of the two sets of runs indicates that the Peng-Robinson package must produce an anomoly in the Gibbs function so the RGIBBS module finds a very strange stationary point that looks like the equilibrium.
RSTOIC can be used to reproduce the same condition if we specify the same extent of the reaction: 0.9045.
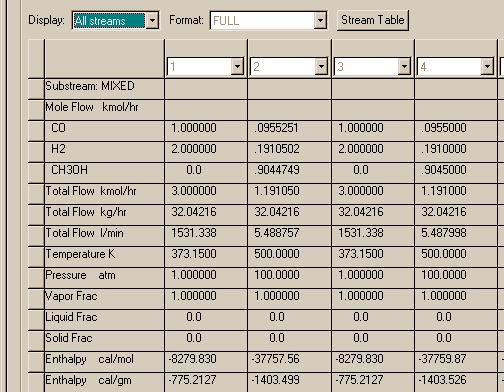
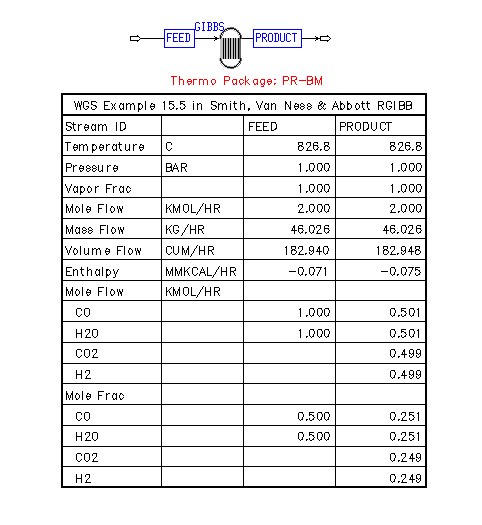
Here are the results of simulating the Water Gas Shift reactor in Aspen. Only one case will be shown: the first problem posed in Smith, et al Example 15.5. The flow diagram was drawn with a single feed connected to a GIBBS reactor and a single product. The following results were found and reproduced from a PFD file in Aspen. The thermodynamics package: Peng-Robinson equation of state with the Boston-Mathias alpha function was used in the simulation. The results are seen to be almost identical to those found using ideal behavior in Matlab and in the text.