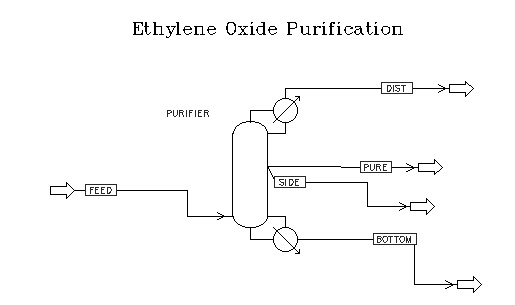
The goal of this project was to design an ethylene oxide purification system. When ethylene oxide is produced by catalytic partial oxidation of ethylene in aqueous phase, the major by-products are carbon dioxide, acetaldehyde, and formaldehyde. The carbon dioxide is removed in an absorber / stripper system; however, the process stream leaving this system still contains water, acetaldehyde, and formaldehyde. This stream must then enter a purification system that removes these contaminants, producing a stream of ethylene oxide that meets a certain purity. The purity goal of this project was 99.95%.
The above separations can be achieved in a single distillation column. This fact is supported not only by ASPEN modeling, but also by existing purification systems in various ethylene oxide plants. Furthermore, it was the conclusion of this project that a column containing fifty trays and a recycle ratio of six would produce an excellent separation. The process feed enters this column at tray forty-three (numbered from the top). The bottoms flow contains virtually all of the water in the system. The distillate contains both ethylene oxide and all the formaldehyde in the system. Because of the number of components in the system, additional product streams are necessary. A small stream containing ethylene oxide and most of the acetaldehyde is taken from tray 40. Most importantly, the high-purity product stream will be taken from tray five.
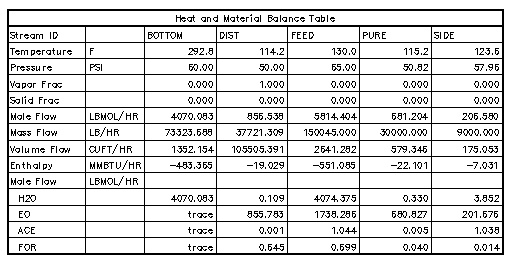
The modeling for this project was done in ASPEN Plus, using the RADFRAC module to simulate the column. Because rust catalyzes a aldehyde-producing reaction, the column should be constructed exclusively of stainless steel. ASPEN estimates the entire cost of this system as $398300. The purity goal of 99.95% EO in the high-purity product stream was met. This stream has a mass flowrate of 30,000 lbs/hr.

Ethylene oxide is an essential building block in numerous chemical processes. Its most common method of production is through the catalytic partial oxidation of ethylene. This reaction is carried out in aqueous phase. Without the catalyst, the predominant reaction would be the complete oxidation of ethylene oxide to carbon dioxide. Even with the catalyst a significant amount of carbon dioxide forms. The yield of CO2 increases as the catalyst ages. Other impurities formed in this process are acetaldehyde and formaldehyde. The reactor effluent is fed to a CO2 stripper/absorber system, which effectively removes all the carbon dioxide from the process stream. Because carbon dioxide is the only by-product whose yield depends on the age of the catalyst, the composition of the stream leaving the stripper/absorber system is relatively independent of catalyst age. On a mass basis, this stream is roughly half water and half ethylene oxide, with minor amounts of acetaldehyde and formaldehyde. The ethylene oxide in this stream must then be purified.
The purpose of this design project was to design a purification system that would separate the water and the aldehydes from the ethylene oxide, producing a product stream that was roughly 99.95 percent ethylene oxide. The composition of the feed stream to this system was based on data obtained through Jeff Anderlik1 at the Celanese Corporation.
An early decision was made to attempt to accomplish this purification using only one tower. According to Anderlik, prior to ten years ago, the Celanese EO Plant in Clear Lake used three separate towers for this separation. However, for the past ten years, the purification has been carried out in a single tower with approximately sixty-five trays. In this tower, the bottoms stream should be exclusively water, while the tops stream will contain most of the formaldehyde, as these components are the least and most volatile in the system, respectively. Thus, two additional side product streams are required: a low-purity stream containing almost all of the acetaldehyde, and a high-purity stream containing approximately 99.95% ethylene oxide. This project will aim to optimize the total number of trays in this column, as well as the location of all feed and product streams.
Another consideration for the design of the tower is the possibility of feeding a small amount of water to the column in addition to the regular feed. The tower in the Celanese plant has the capability to do this; however, the performance of their tower has been good enough that the additional water feed has not been necessary. In theory, since acetaldehyde can dissolve in water, this addition might improve the separation of ethylene oxide and acetaldehyde. However, this water addition could also increase the amount of water in the product streams. This project will investigate the use of an additional water feed, and possibly include this system in the final tower design.
Before any modeling of the tower in ASPEN Plus could be attempted, a package of thermodynamic data had to be selected. The first step in this process was to find relevant experimental vapor-liquid equilibrium data. Data from two binary systems, ethylene oxide-water and ethylene oxide-acetaldehyde, was obtained for this purpose2. The second step involved running equimolar mixtures of each of these two systems through a flash module in ASPEN, using a number of different thermodynamic packages. These packages included UNIFAC, Wilson, NRTL, and ideal. The ASPEN results corresponding to each of these packages were then compared to the experimental data to determine the best package for the relevant components.
The first binary system analyzed was the ethylene oxide-water system. The vle data used was isobaric at one atmosphere. As the feed to the column is roughly half water and half ethylene oxide (on a mass basis), the thermodynamic package selected should match the experimental data of this binary system rather closely.
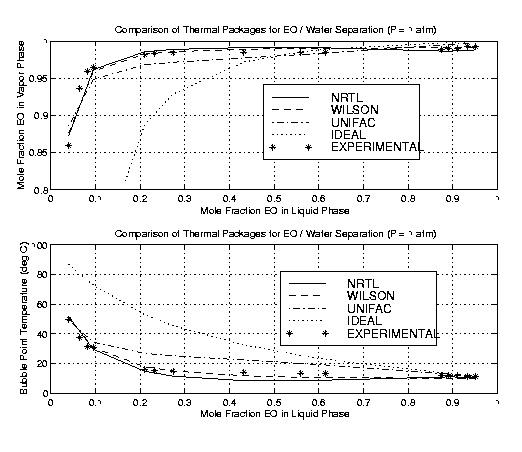
It is immediately obvious that this system deviates greatly from ideality, and that the ideal package would thus be inadequate. The UNIFAC results were also inaccurate, but were much closer than the ideal results. Both the Wilson and the NRTL package produces good results for this system, and both were strong possibilities for the eventual choice of thermodynamic package.
The second system analyzed was the ethylene oxide-acetaldehyde system. This system represents the most difficult separation in the tower: the EO / acetaldehyde separation. Although there is not nearly as much acetaldehyde as water in the column, most of the trays in the column are promoting this separation. Once again, the data was isobaric at one atmosphere.
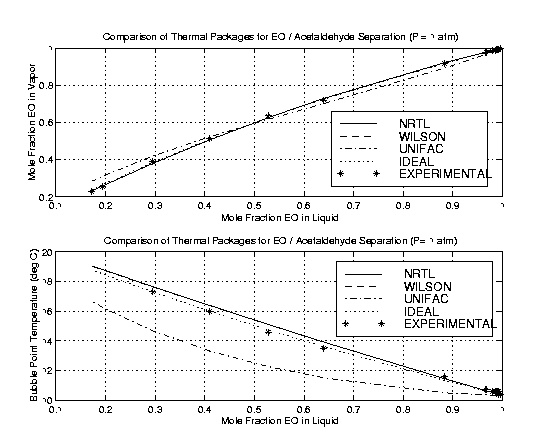
In this case, surprisingly, the ideal package produced good results. UNIFAC's results were the furthest from the experimental. Once again, both Wilson and NRTL produced very satisfactory results. Based on comparisons to experimental data, it was concluded that either NRTL or Wilson would be used in ASPEN modeling for the tower.
The final decision as to which package to use did not occur until both were tried in preliminary models of the tower in ASPEN. The Wilson package consistently caused errors, while the NRTL package ran rather smoothly. As both packages were considered acceptable based on experimental data, it was decided to select NRTL as the thermodynamic package for the modeling in ASPEN.
The first major design decision was the number of trays to include in the column. Using sensitivity runs in ASPEN Plus, the dependence of the purities of the product streams on the total number of stages could be readily plotted. Existing towers in industry, such as Celanese's, have accomplished the desired purification in roughly sixty-five stages. Sixty-six was taken as the upper limit as the number of stages in the new column. The lower limit for number of stages was set at forty-three, because any number lower than this produced errors in ASPEN. The purity of all three product streams (the distillate, the high-purity, and the low-purity) was considered.
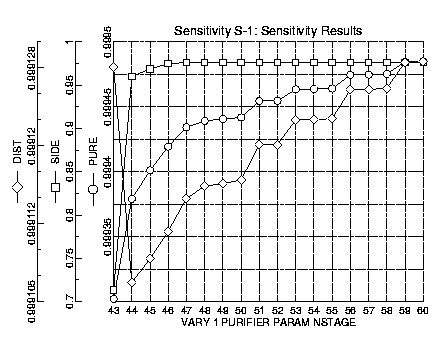
It is obvious that the purities of each of the product streams approaches a limit as the number of stages increases. After roughly the forty-eighth stage, the increase in the purities is minimal, as can be simply seen by noticing the y-axis scaling on the previous plot. However, it was still somewhat unclear whether it would be financially advantageous to increase the number of stages over fifty. To finally determine the number of stages, RADFRAC runs were made with 40, 45, 50, and 66 total stages.
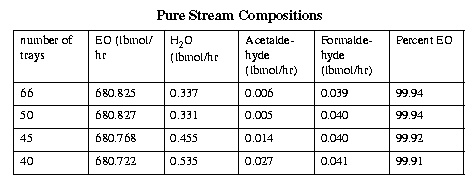
These runs indicate that there is no advantage to increasing the number of stages above 50. However, there is a noticeable decrease in performance when the total number of trays is lowered to 45 or 40. There is some minor disagreement between the stream compositions in the sensitivity runs and the actual RADFRAC runs. These discrepancies are probably due to the fact that the sensitivity runs only allow one to vary one parameter (in this case total number of stages), while RADFRAC runs are generally done varying all the stream locations. All these differences are on the order of a thousandth of a percent, and thus negligible. It was decided to use fifty trays in the purification column.
After determining the number of trays in the column, the recycle ratio had to be specified. Since only approximately one sixth of the moles entering the column exit through the distillate, a high recycle ratio will not lower the net flow through the column as much as it would if the distillate flow was larger. ASPEN's DISTWU module was used to predict the recycle ratio required to separate ethylene oxide from acetaldehyde in sixty trays. It required a reflux ratio of just over 3.9. However, the column in question would have fewer trays, and would not have the acetaldehyde exiting through the bottoms. Because of the complex nature of this column, the reflux ratio could only be determined through trial and error, starting with 3.9 and increasing slowly until the purity of the products streams met their goals. It was determined that a reflux ratio of six produced streams that met the purity goals.
Once the number of trays and the reflux ratio were determined the task became locating the feed and side streams and determining stream flow rates. As water is the least volatile of the components it will virtually all leave the column through the bottom stream. As the feed contained seventy percent by mole water, the bottoms flow was specified to be seventy percent of the feed. Once the bottoms to feed ratio and the reflux ratio were specified sensitivity runs could be performed to determine the best location for the feed and side streams.
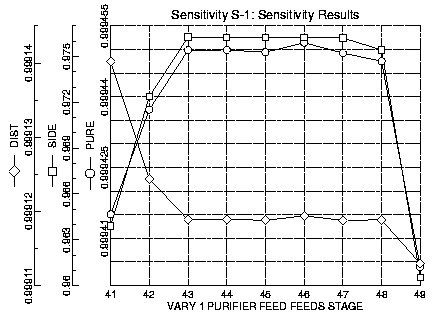
The results of varying the feed location to optimize the pure product stream indicate that the feed should be located from stages forty-three to forty-seven. It was determined that the column would have more difficulties with flooding if the feed stream entered near the bottom of the column. From these results the feed was located at stage forty-three. The flow rate of the feed was set to 150054 lb/hr, the feed flow rate of the column in the Celanese plant.
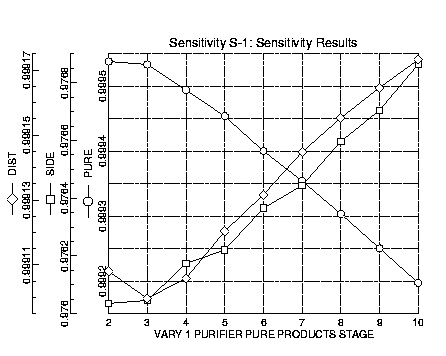
The desired location of the pure stream was known to be very high in the column. Ethylene oxide is more volatile than acetaldehyde and water but less volatile than formaldehyde. Most of the formaldehyde goes out the top in the distillate stream, creating a relatively pure stream with minor amounts of formaldehyde. The results of the sensitivity run for the location of the high-purity stream indicate that the purity of this stream increases with increasing height in the column. However, to avoid drying of plates in the upper portion of the column, the high-purity stream was located at tray five At this location the column has two relatively pure streams, the high-purity side stream and the slightly less pure distillate stream. The flow of the side stream was maximized in RADFRAC such that the column did not dry and convergence was achieved. The final specification for the flow of the pure stream was 30000 lb/hr.
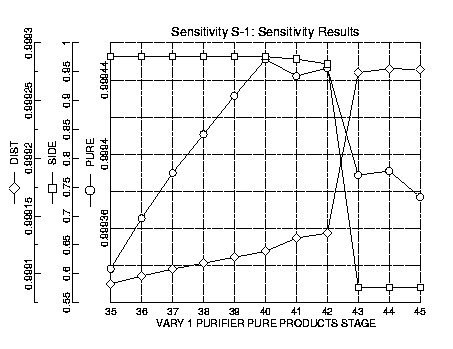
Finally the location of the lower-purity side stream was determined. Knowing the relative volatilities of the components, this side stream would be most effective at acetaldehyde removal at a lower stage. Thus sensitivity runs were performed with the stream location varying from plate thirty-five to plate forty-five. The purity of the product streams steadily increased until it reached a maximum at stage forty. At this point the purity decreased as the side stream location neared the feed location at which point a marked decline occurred. As a result the side stream was located at stage forty. The flow through the side stream needed to maximize the amount of acetaldehyde removed and minimize the amount of ethylene oxide lost. Through rigorous trial and error methods the optimum flow was determined to be 9000 lb/hr.
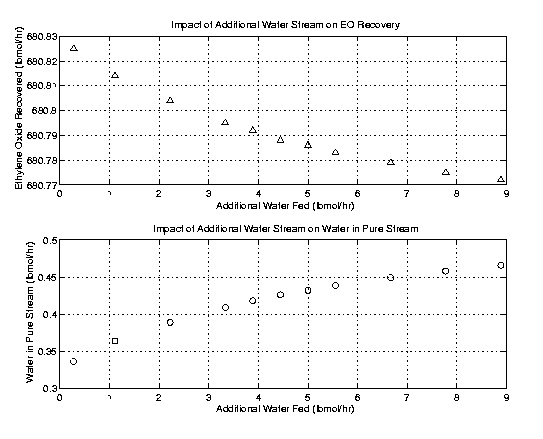
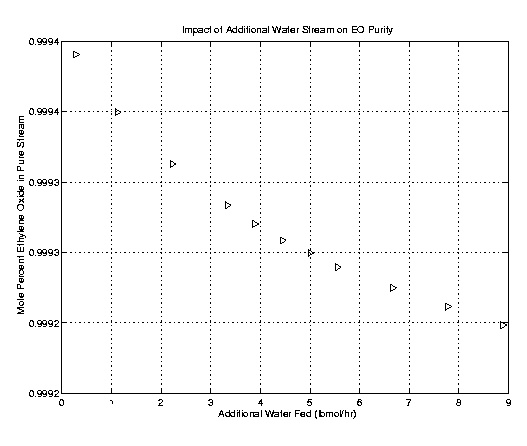
Once the column was optimally designed the issue of an additional water stream was addressed. The theory presented was that acetaldehyde removal could be facilitated by an additional water input. Acetaldehyde is more soluble in water than in ethylene oxide. As a result the additional water stream would theoretically remove more acetaldehyde. Using the fifty stage column outlined above, several runs with varying water flow rates were executed and compared. As the following plots indicate the addition of a water stream does little to improve the high purity stream. In fact it actually decreases the purity of the product. The reduced purity stemmed from an increase in water and a decrease in ethylene oxide in the product stream. Further, acetaldehyde and formaldehyde removal was not improved. These results lead to an omission of the water stream. However, it is recommended that the additional water stream be kept in mind for flows with higher concentrations of acetaldehyde.
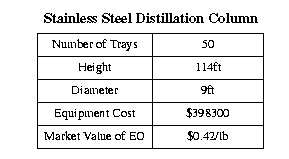
The recommended operating conditions for this tower are based primarily on those of existing towers. In general, the pressure of the reboiler (stage fifty) of the column should be sixty psi. The pressure should drop slightly as one moves up the column, and the condenser should be run at fifty psi. The temperature corresponding to these pressures average about 120 degrees Farenheit above the feed, and about 290 degress below the feed. See Appendix A for detailed temperature and pressure profiles in the column. The designed column measures 114 feet, tangent to tangent. It must not contain any carbon steel, as nickel catalyzes a reaction that produces various aldehydes. The equipment cost of the tower was estimated by ASPEN to be $398300.
1) Anderlik, Jeff. Personal correspondance.
2) Chu, Ju Chin. Vapor-Liquid Equilibrium Data, J.W. Edwards Publisher, Inc., Ann Arbor, Mi 1956