ABSTRACT
A network of heat exchange using process streams was designed for an ethanolamine production plant. Aspen and Matlab were used to generate the results. Savings estimates were based on the elimination of stem in the system. An annual savings of $195,292 was predicted. Safety and feasibility were also considered and were found to be above satisfactory. It was found, using Matlab, that temperature differences were no less than 50°C in each heat exchanger. A vertical shell-and-tube heat exchanger was rigorously designed in Aspen for exchange between the main column reboiler and the first compression stage exit stream. The optimal exchanger design was based on efficiency of heat exchange and pressure drop along the exchanger. The exchanger was designed to be 16 feet long and 4.6 feet in diameter, with 100 4-inch diameter tubes constructed of stainless steel. It is recommended that the designed heat exchange system be implemented in the ethanolamine production process.
INTRODUCTION
The purpose of this production process is to convert ethylene oxides and ammonia into ethanolamines, namely monoethanolamines (MEA), diethanolamines (DEA) and triethanolamines (TEA). Although MEA is the most profitable, all three products are in great demand, and thus the process not only requires a versatile reactor design, but also a high purity separation. Yet in order for the entire process to run successfully, the appropriate streams must be either heated or cooled before entering reactors and/or separators. This is why heat exchanger design is an integral part of our system.
The heart of this design lies in the theory of "hidden sources of heat," stemming from the use of compressors to avoid the high cost of steam. As seen in the heat exchanger network, there are many streams which need to either be heated or cooled and therefore this modified system results in a significant cost savings.
PROCESS OVERVIEW
An ethanolamine plant consists of three sections: compression, reaction, and separation. Raw materials cycle through these sections in a loop as a water/ammonia mixture is compressed to a high pressure, reacted, and brought back to atmospheric pressure for separation. There are 10 points within the process where a stream must be heated and cooled.
Reaction
The reactors accept a stream of pressurized (69 atm), hot (125°C) ammonia mixed with water. The first reactor heats this stream to 178°C, and boils off some of the ammonia in the process. The reactor heat exchanger must cool this stream back to 125°C, where it can enter a second reactor. The second reactor effluent is fed directly to the separations section.
Separation
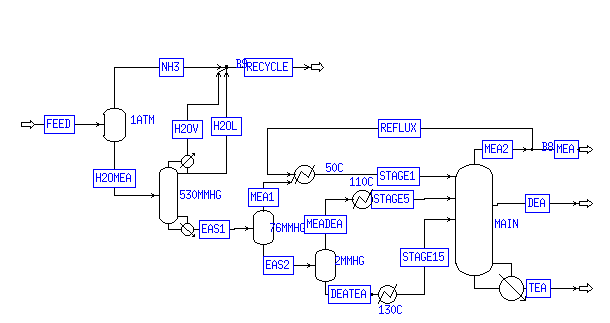
Because the majority of heat exchange occurs in the separation section of the process, only this process was modeled for the purposes of heat exchange. Figure 1 details the separation process.
Figure 1: Separation Process

The effluent from the second reactor is first passed through an unheated flash vessel. The stream is then fed to the water/ammonia column. The column includes a reboiler running at 177°C and a condenser running at 50°C. The vapor product is mixed with additional ammonia and water and recycled to the compressor section of the plant.
The bottoms of the column, consisting almost entirely of ethanolamines, are flashed at 85°C and 7.6 mm Hg. This flash tank, or the stream entering the tank, must be cooled slightly to keep the temperature at the right value. Much of the monoethanolamine evaporates, and the liquid phase of roughly 1/3 MEA, 1/3 DEA, and 1/3 TEA is sent to the main distillation column.
The 20-stage main column requires two feeds; one at stage 5 of largely MEA and DEA, and one at stage 15 of largely DEA and TEA. A flash vessel at 2 mm Hg and 121°C performs this separation. The stream immediately before the flash vessel, or the flash vessel itself, must be heated to keep the temperature at this value. The MEA/DEA mixture in the vapor phase must be cooled slightly, to 110°C, before being fed to stage 5. The DEA/TEA mixture must be heated slightly, to 130°C, before being fed to stage 15. The exact temperature of these two streams is an important parameter in controlling the output of the column.
UTILITIES COSTS
To compare equipment and operating costs, we first consider a case where only utilities are used for heat exchange. This configuration could result in lower equipment costs than using process heat exchangers, but it will require high operating costs.
Heating
There are four process streams that require heating. Table 1 outlines the heating requirements of the process.
Table 1: Heating Requirements
|
Process |
Streams |
Steam |
||||
|
Stream |
From |
T (oC) |
To |
T (oC) |
kg/hr |
psi |
|
C1 |
Water/ Ammonia Column |
164 |
Reboiler |
177 |
3384 |
170 |
|
C2 |
Main Column |
173 |
Reboiler |
177 |
841 |
170 |
|
C3 |
Rough Ethanolamine Flash |
121 |
Main Column |
130 |
42 |
70 |
|
C4 |
MEA Separation Flash |
85 |
Rough Ethanolamine Flash |
140 |
1400 |
70 |
To minimize the amount of time that the process stream spends in contact with the reboiler, a vertical thermosyphon should be employed for each reboiler.
The Oxychem ethanolamine plant in Clear Lake uses 250 psi steam in the high-temperature column reboilers, as opposed to the choice of 170 psi used here, and 100 psi steam for lower-temperature heating. The plant generates 250 psi steam, passes it through a reboiler, and then flashes the condensate at 100 psi to generate the lower-pressure steam. Instead of building two steam generators, this may be a more cost-effective equipment setup for this process as well.
Cooling
There are six process streams that must be cooled. In each case, cooling water is assumed to be available at 300 K (80°F), and cooling water is allowed to warm to a temperature between 321 K (118°F) and 325 K (125°F). If a higher cooling water temperature is allowed, then the flowrates shown can be reduced. If cooling water is allowed to warm to only 312 K, for instance (102°F), the flowrates here can be roughly halved.
Table 2: Cooling Requirements
|
Process |
Streams |
Cooling Water |
|||
|
Stream |
From |
T (oC) |
To |
T (oC) |
kg/hr |
|
H1 |
MEA Separation Flash |
94 |
Main Column |
50 |
42,894 |
|
H2 |
Water/NH3 Column |
76 |
Condenser |
50 |
21,060 |
|
H3 |
Rough Ethanolamine Flash |
121 |
Main Column |
110 |
2096 |
|
H4 |
Water/NH3 Column |
177 |
MEA Separation Flash |
167 |
1763 |
|
H5 |
First Stage Compressor |
321 |
Second Stage Compressor |
40 |
122,960 |
|
H6 |
Second Stage Compressor |
294 |
Recycle |
105 |
148,500 |
Aspen models were used to calculate these figures. Because cooling water is likely generated within the plant using a cooling tower, the cost of the cooling water is based largely on the equipment cost of the cooling tower and related equipment. The design estimate outlined here does not attempt to calculate a cost for the cooling water required.
The cost of steam will also vary widely depending on its source and proximity. If the ethanolamine plant is located near a refinery or other facility that produces excess steam, the steam may be purchased at a very favorable rate. For the purposes of this design estimate, a set of assumptions is used to find the lowest possible cost of steam.
First, the heat exchangers and piping are assumed to be perfectly insulated. In addition, there is no pressure drop on the steam side of the heat exchangers. The steam is produced using two on-site boilers, each burning natural gas. The condensate from the 70 psi steam heat exchangers is fed to the first boiler, and the 170 psi condensate is fed to the other. The two boilers are assumed to be 100% efficient, and every BTU of energy stored in the natural gas is released in the boiling.
With these assumptions, the total energy fed into the boiler is equal to the total amount of energy required by the heat exchangers, 2.623 mmkcal/hr. If the price of natural gas during the first year of operations is roughly equal to the current price of natural gas futures (about $2.20/mmscf), and the heat content of the natural gas is the same as the average heat content of natural gas taken from the United States last year (1028 BTU/ft3), then the plant will incur costs of $8.49 per mmkcal of heating required. For the required 22,976 mmscf, the minimum annual steam cost will be $195,292. In practice, the steam boiler will not be perfectly efficient, the pressure drop in the exchangers will not be zero, and the price of natural gas may rise far above the $2.20 quoted - in short, the actual price of steam will significantly exceed the $195,292 figure.
HIDDEN SOURCE OF HEAT
It is apparent from the utility cost analysis that the greatest utility cost is due to the steam required for heating in the separation process. In a study of the separation process, a "hidden" source of heat was discovered.
As described in the process overview, the effluent from the second reactor is first flashed and then distilled in order to remove the water and ammonia present in the stream. This water and ammonia are then sent back to the first reactor as a recycle stream. The flash and distillation column are run at 1 atm and 530 mm Hg, respectively. The first reactor is run at a pressure of 69 atm. Therefore, the recycle stream must be compressed before it is fed into the first reactor.
The compression of this stream was first modeled as a single-stage compressor. This resulted in a temperature increase of approximately 1000°C. In order to avoid damage to the compressor, this model was rejected in favor of a two-stage compression system. This resulted in the first and second stages being heated to 321 and 294°C, respectively.
It was assumed that the stream was cooled to 40oC after the first stage of compression and to 105°C before being recycled to the first reactor. The system is shown in Figure 2.
This compression system is necessary for process and safety reasons. The two streams involved require a great deal of cooling, and they can be used to provide a large amount of heat when incorporated into a heat exchange network. These two streams are essential to the heat exchange network presented here, and they are also the source of the greatest amount of savings generated by this network.
HEAT EXCHANGE NETWORK
Analysis of Process
Significant utilities savings can be attained by implementing a network of heat exchange among the process streams. First, an analysis was done to determine the feasibility of heat exchange in the ethanolamine plant. This analysis was done in two steps: first, the reaction process and second, the separation process.
The reaction process includes only one stream that could be involved in heat exchange. The effluent from the first reactor, which is fed to the second reactor, must be cooled from 178°C to 125°C. This stream was ultimately not included in the heat exchange network for two reasons. First, there is more cooling required in the separation process than can be attained using heat exchange. Therefore, some cooling water must still be used in the overall process. Second, the reactor stream is at a pressure of 69 atm. Since most process streams are below 1 atm, safety considerations prove cooling water to be a better choice for this stream.
The results of the separation process analysis were a system of four cold streams that require heating and six hot streams that require cooling. Figure 3 provides a visual representation of the heating and cooling required in the system. Refer to Tables 1 and 2 for descriptions of the streams.
Figure 3: HENS Diagram

From this figure, it is easy to see that streams H1 and H2 will require the use of cooling water, as cold streams are not present at temperatures suitable for heat exchange.
Development of Network
With the removal of streams H1 and H2, the final heat exchange network includes four cold streams and four hot streams. The cold streams can be completely heated using only the hot process streams available. The heat exchange network includes all cold streams from the entire process. Therefore, the need for steam is entirely eliminated. The hot streams are cooled as much as possible using the cold process streams, and the remaining cooling needs are met using cooling water.
Figure 4 shows this heat exchange network pictorially. The points of heat exchange are shown in color to make understanding the process easier. The hot streams (which require cooling) are in red, and the cold streams (which require heating) are in blue. The Hot streams are color-coded, and each hot streams is used to heat the cold stream(s) of the corresponding color.
Figure 4: Heat Exchange Network
Table 3 details the network of heat exchange in the separation process and includes the duty for each heat exchanger.
Table 3: Heat Exchanger Functions
|
First Stage Compressor Aftercooler (321°C - 40°C) |
||
|
Rough Amine Separation Flash (85°C - 140°C) |
0.6519 |
mmkcal/hr |
|
Ethanolamine Column Reboiler (173°C - 177°C) |
0.4 |
|
|
Cooling Water |
1.7381 |
|
|
2.79 |
||
|
Second Stage Compressor Condenser (294°C - 105°C) |
||
|
Water/Ammonia Column Reboiler (164°C - 177°C) |
1.55 |
mmkcal/hr |
|
Cooling Water |
2.026 |
|
|
3.576 |
||
|
MEA Separation Flash (177°C - 167°C) |
||
|
Ethanolamine Stage 5 Heater (121°C - 130°C) |
0.020884 |
mmkcal/hr |
|
Rough Ethanolamine Flash (85°C - 140°C) |
0.024616 |
|
|
0.0455 |
||
|
Ethanolamine Stage 5 Cooler (121°C - 110°C) |
||
|
Rough Ethanolamine Flash (85°C - 140°C) |
0.05764 |
mmkcal/hr |
|
Cooling Water Alone Used |
||
|
Ethanolamine Column Condenser |
0.9531 |
mmkcal/hr |
|
Water/Ammonia Column Condenser |
0.5988 |
Utility Savings
This heat exchange system results in a complete elimination of steam in the process. Since the utilities costs analyzed here were based solely on steam, the system results in a 100% utilities savings. In fact, even more savings are present because of the reduction of cooling water needs; however, these savings are small compared to those gained by eliminating the use of steam. Therefore, the savings based on this heat exchange network is $195,292 annually.
Feasibility
Two very important factors were analyzed to determine the feasibility of the heat exchange network. First, pressure differences among the streams were examined. This is an important consideration because much of the separation process is run at low pressures.
It was determined that the heat exchange process is actually much more favorable than the use of utilities when pressures are considered. This is due mainly to the fact that the steam required to cool the hot streams would be at a much higher pressure than the hot process streams used in the heat exchange network. The lowest pressure steam required would be at 70 psi, and the highest pressure steam would be at 150 psi. In contrast, the hot process streams are at much lower pressures. As a result, using heat exchange is not only more efficient, but also safer than using utilities in this process.
The second factor examined in the feasibility determination was temperature differences in each heat exchanger. Of course, in each heat exchanger, the hot stream must never become colder than the cold stream and vice versa; both are physical impossibilities. However, it is also desirable to maintain the temperature difference above a minimum value, generally given as 10°C.
The Matlab program htxcc1 was used to detail the temperature differentials of each heat exchanger. The program generates a plot of the temperature of each stream and the difference in temperature along the length of a single pass, shell-and-tube heat exchanger. It was found that the heat exchange which had the smallest temperature difference was between the rough ethanolamine separation flash and the first stage compressor. The temperature plot is shown in Figure 5.
Figure 5: Minimum Temperature Differential

It can be seen from this figure that the minimum temperature difference in the exchanger is between 50 and 100°C. Nowhere in the heat exchange network is the temperature difference any smaller. Therefore, based on pressure and temperature differences in the heat exchangers, the system was determined to be feasible.
Control
While the design of this heat exchange network focuses on achieving the most efficient recycling of heat within the plant, plant operators still retain control over the exact temperature and duty of each heat exchanger. Each stream passes through either an exchanger cooled with cooling water, or with a process stream that is itself cooled by cooling water. The exchangers appear to be in a delicate balance with each other, but that balance can be changed at will to accommodate new situations.
For example, changes in the desired product distribution may require that the column feed at stage 5 be cooled to 108°C, instead of the current 110°C. This stream is cooled by exchanging its heat with the cooler stream entering the heated flash, and the new temperature setpoint will result in a warmer heated flash stream. The heated flash stream is also passed through the first stage compressor aftercooler, however, and reducing the heat transferred from this stream will keep the heated flash at the correct temperature. Ultimately, the compressor aftercooler, which is cooled with cooling water, will need an increase in the flowrate of cooling water to keep it at the correct temperature. By adding more cooling water at one point in the process, and changing the heat exchanged with the process heat exchangers, the temperature of any process stream can be adjusted precisely.
CRITICAL HEAT EXCHANGER
The critical heat exchanger which was chosen was the MEA Reboiler to be used in conjunction with Compressor 1. As previously described, this "exchanger system" is key to the heat exchange and control of our process.
Design
The exchanger was designed to be a shell and tube heat exchanger with 100 tubes. The tubes are 4 inches in diameter. This is larger than most standard heat exchanger tubes. The choice was made to use 4 inch tubes because very high velocities resulted when smaller tubes were used. This would result in an undesirable pressure drop across the reactor.
This critical heat exchanger was chosen to be vertical since ethanolamines will decompose if exposed to heat for an extended period of time, as they would be in a horizontal exchanger. The dimensions of the exchanger, constructed out of stainless steel, are 4.6 feet in diameter and 16 feet long, due to the velocity distribution obtained at these sizes.
Cost and Savings
Aspen costed the equivalent carbon steel exchanger at $17,800. It was assumed, based on industry standard, that a heat exchanger made of stainless steel would have twice the cost. Thus, the Aspen-based cost of this heat exchanger is $35,600.
A comparable heat exchanger was also found at Union Carbide for a lower price. Their vertical carbon steel heat exchanger, priced at $10,000, had very similar dimensions to the one designed in Aspen and consisted of over 1000 tubes. It is estimated that an exchanger with about 100 tubes would be significantly cheaper. If $10,00 is used as a base cost, however, a stainless-steel exchanger would have an estimated cost of $20,000. This demonstrates the low cost of the heat exchanger when compared to the utilities savings and also verifies Aspen's relative costing accuracy.
Overall, this exchanger would result in an annual steam savings of $30,000. Thus, the installation of this heat exchanger would result in considerable utilities savings over the life of the plant.
CONCLUSIONS
This heat exchange design for an ethanolamine plant will result in great utilities savings. The majority of this savings stems from the removal of high-pressure steam in the separation process. Hot streams from the required recycle compression are used to heat the cold streams in the process, and the cold streams are, of course, used to cool the hot streams. A savings of $195,292 is generated, based solely on the elimination of stem in the system.
In addition to cost savings, the implementation of heat exchange is beneficial for two more important reasons. First, the network presented here maintains precise control of the process. In addition, exchanging heat among process streams will eliminate the safety hazards of using high-pressure steam to heat a low-pressure system.
For the reasons stated above, the heat exchange network presented here should be implemented in the construction or revamp of an ethanolamine plant. The results of the system show that heat exchange is a viable and beneficial option in ethanolamine production.