![[Maple Math]](images/402_project1.gif)
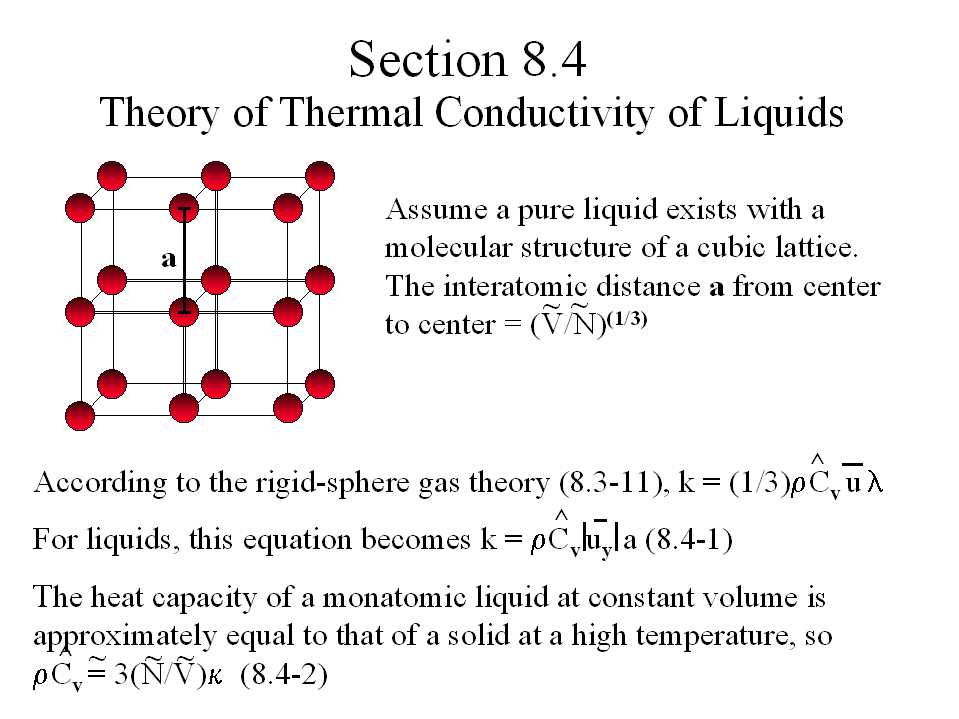
> restart;
> rho:=1.595*g/cm^3; dpdrho:=90.7*10^(-6)/atm; mass:=153.84*g; Navogadro:=6.023*10^23; kappa:=1.3805*10^(-16)*g*cm^2/s^2/K;
![[Maple Math]](images/402_project1.gif)
![[Maple Math]](images/402_project2.gif)
![]()
![]()
![[Maple Math]](images/402_project5.gif)
where rho is the density of the liquid, dpdrho is the compressibility, mass is the molar mass, Navogadro is Avogadro's number, and kappa is Boltzmann's constant.
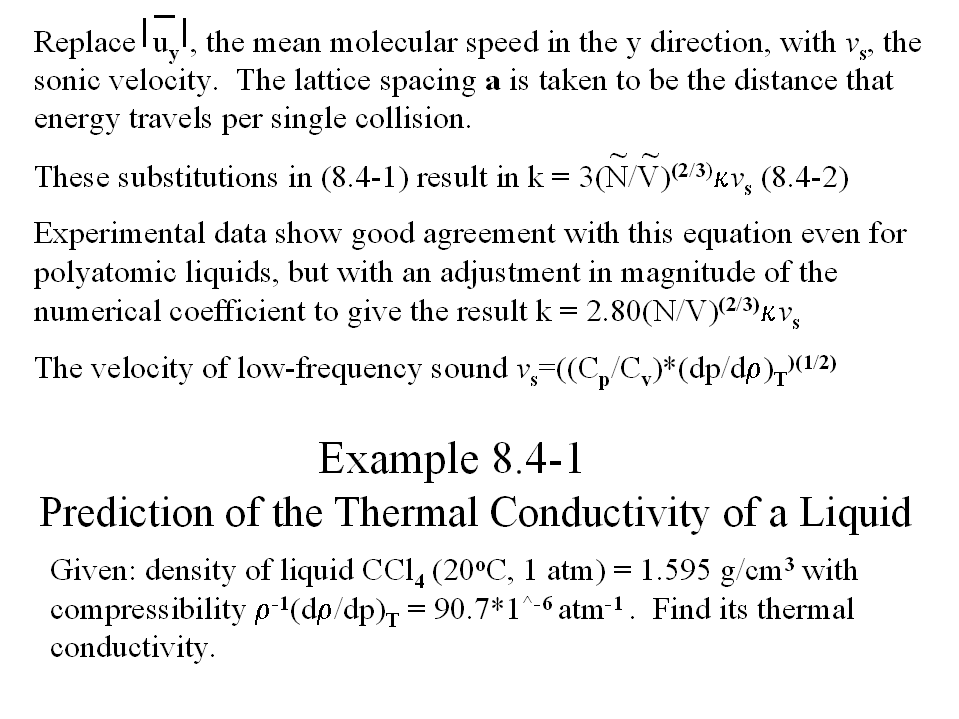
Set variable eta = (dp/drho) at constant T.
Since (dp/drho) at constant T = 1/(rho[rho^(-1)*(drho/dp)Tconst]),
> eta:=1/(rho*dpdrho);
![[Maple Math]](images/402_project6.gif)
> eta := 6912.450359*cm^3*atm/g;
![[Maple Math]](images/402_project7.gif)
> atm:=1.1033*10^6*g/cm/s^2; define atm in different units in order to simplify expression
![[Maple Math]](images/402_project8.gif)
> eta; simplified units of (dp/drho)Tconst
![[Maple Math]](images/402_project9.gif)
Set variable beta = Cp/Cv and assume its value to be 1.0.
> beta:=1.0;
![]()
> vs:=(beta*eta)^.5; equation (8.4-4)
![[Maple Math]](images/402_project11.gif)
> assume (cm>0);
> assume (s>0);
> simplify (vs);
![[Maple Math]](images/402_project12.gif)
> Vmolar:=mass/rho;
![]()
> k:=2.80*(Navogadro/Vmolar)^(2/3)*kappa*vs;
>
![[Maple Math]](images/402_project14.gif)
> simplify(k);
![[Maple Math]](images/402_project15.gif)
> changeunitsvar1:=2.3901*10^(-8)*cal/s/cm/K/g/cm*s^3*K;
![[Maple Math]](images/402_project16.gif)
> k2:=k*changeunitsvar1;
![[Maple Math]](images/402_project17.gif)
> simplify(%);
![[Maple Math]](images/402_project18.gif)
> simplify(%,assume=positive);
>
>
>
![[Maple Math]](images/402_project19.gif)
> unassume(s>0);
![]()
> k2;
![[Maple Math]](images/402_project21.gif)
> simplify(k2);
![[Maple Math]](images/402_project22.gif)