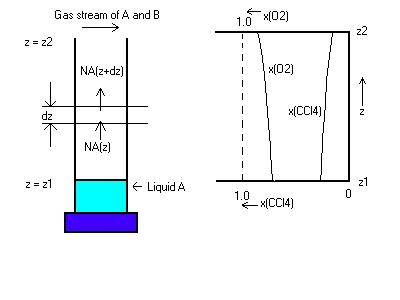

> restart; Example 17.2-1: Determination of Diffusivity
Begin by defining constants, such as the density, time, temperature, distance travelled by the CCl4, etc.
> MWCCl4:=1/154*gmole/g; Area:=.82*cm^2; t:=3600*10*s; rhoCCl4:=1.59*g/cm^3; R:=82.05*cm^3*atm/(gmole*K); T:=273*K; dz:=17.1*cm;p:=755/760*atm; pO2_2:=755/760*atm; pO2_1:=722/760*atm; volrate:=0.0208*cm^3;
![]()
![]()
![]()
![[Maple Math]](images/402projmath4.gif)
![[Maple Math]](images/402projmath5.gif)
![]()
![]()
![]()
![]()
![]()
![]()
> NCCl4:=volrate*rhoCCl4/(Area*t)*MWCCl4; Determine the molar flux of CCl4 using the volumetric evaporation rate.
![[Maple Math]](images/402projmath12.gif)
> DCCl4_O2:=NCCl4*dz*R*T/(p*ln(pO2_2/pO2_1)); Determine diffusivity using equation 17.2-15a in the book.
![[Maple Math]](images/402projmath13.gif)
> simplify(%);
![[Maple Math]](images/402projmath14.gif)
>