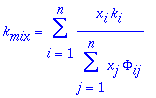
Thermal conductivity of a gas mixture is estimated by:
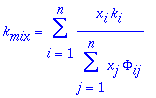
where xi are mole fractions and ki are thermal conductivity for pure components. The F function is as follows:
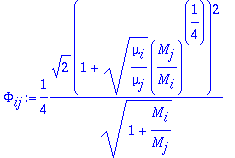
The MATLAB program mixkt calculates the thermal conductivity given thermodynamic properties of each component.
function kt = mixkt(zs,T,j) % mixkt(zs,T,j) finds the thermal conductivity of a % low density gas mixture % zs gives the mol fraction of each compound % T gives the temperature in the units of Tdeg % j gives the indices of the compounds (if missing it includes % all compounds.) zs must be consistent with j. % Based on equations 8.3-17 & 18 in BS&L % The thermal conductivity is given in W/m/K global mw if nargin<3 j=1:length(mw); end phi=mixphi(T,j); kts=ktpcalc(T,j); den=phi*zs'; kt=zs*(kts./den); % This file was created by m2html.
Predict the thermal conductivity of the following gas mixture at 1 atm and 293 K based on data on the pure components at 1 atm and 293 K:
|
Species |
i |
xi |
Mi |
mix107 (g cm-1 sec-1) |
kix107 (cal sec-1 cm-1 K-1) |
|
CO2 |
1 |
0.133 |
44.010 |
1462 |
383 |
|
O2 |
2 |
0.039 |
32.000 |
2031 |
612 |
|
N2 |
3 |
0.828 |
28.016 |
1754 |
627 |
>> zs = [.133 .039 .828]
zs =
0.1330 0.0390 0.8280
>> mixkt(zs,293,1:3)
ans =
0.0231
From convu:
0.2310000E-01W/(m*K) = 0.5521130E-04cal/(cm*s*K)
Since this is an approximation, it is slightly off from BS&L, whose value is 0.584x10-4 cal cm-1 sec-1 K-1.