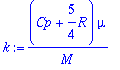
A different formula is required for polyatomic molecules because these molecules have rotational and vibrational energy in addition to the kinetic energy of translation of the center of mass. These forms of energy may be exchanged in collision.
The MATLAB function ktpcalc is used to calculate the thermal conductivity of polyatomic gases at low density using the Eucken approximation:
Where Cp is heat capacity and m is viscosity. This equation also contains the monatomic equation for the case where:
function KTP = ktpcalc (T,index) % Eucken formula for thermal conductivity of polyatomic gas at low density % % function K = ktpcalc(T,index) % % Argument List: % T [=] temperature in the units of Tdeg % index [=] index of compounds in cnms whose coefficients are to be found % (If this argument is omitted, all coefficients will be found.) % Returns: % K [=] heat transfer coefficient in units of W/m/K % % Ex: stationnm% start301a to set compound names & data file % >> start402b to load the data file % >> ktp = ktpcalc(298, 1) % ktp = % 0.0197 Created 3/21/95-2 Jim Lee, corrected 3/16/99 by SHD global mw cpv cnms mu = mucalc(T); T=at(T); [row col] = size(cpv); for counter=1:row if isnan(cpv(counter,:)) fprintf('Heat capacity data for compound *%s* is not available.\n',cnms(counter,:)) disp('Taking heat capacity as 33.314 J/mol/K...') Cp(counter,1) = 33; else Cp(counter,1) = polyval(cpv(counter,col:-1:1),T); end end R = 8.3145; if nargin==1 KTP = (Cp+1.25*R).*mu./mw*1000; else KTP = (Cp(index)+1.25*R).*mu(index)./mw(index)*1000; end % This file was created by m2html.
Estimate the thermal conductivity of molecular oxygen at 300 K and low pressure.
>> ktpcalc(300,2)
ans =
0.0257
From convu:
0.2570000E-01W/(m*K) = 0.6142556E-04cal/(cm*s*K)
This matches very well with the measured value of 6.35x10-5 cal cm-1 sec-1 K-1 and the BS&L value.
We see that the monatomic theory is not valid for O2 by the following:
>> ktcalc(300,2)
ans =
0.0201
This value is quite different from the polyatomic value.