This section looks in depth at a catalytic reactor where a dimerization reaction (2A --> A2) is occurring. For ease of theory, it is assumed that each catalyst particle is surrounded by a stagnant gas film that A must diffuse through. Then, while at the surface, the reaction takes place immediately and the product, A2, must diffuse back out of the film.

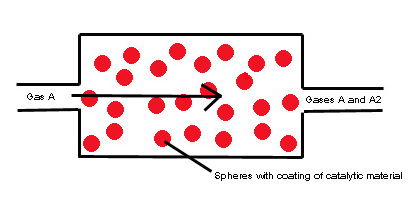
Figure a) This is a view of what is happening at the macroscopic level of the system.
From a quick look at the reaction equation, 2A --> A2, one can see clearly that for each mole of A2 that leaves the film, two moles of A must have entered.
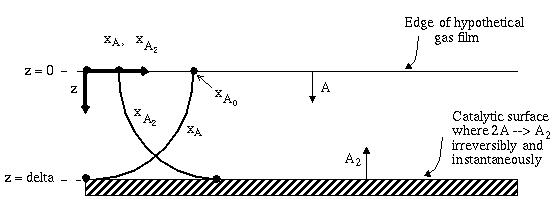
Figure b) This figure represents the model of the activity near the catalyst surface and in the supposed stagnant film.
Now we start the actual equation crunching. The following equations are the basic ones established in the beginning of the chapter:
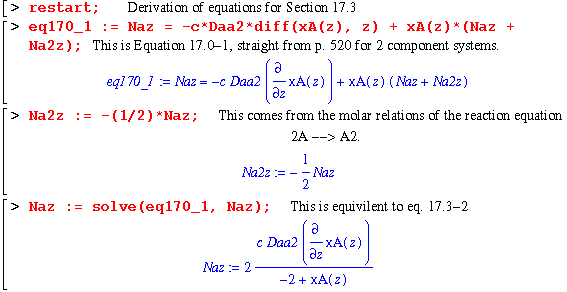
The next section gets us to eq. 17.3-4. Note that constDNaz is an expression with the c and Daa2 terms removed. This is due to the fact that they would disappear in the next step, namely the differentiation of Naz with respect to z (equaton 17.3-3). I remove them in this step to make the worksheet easier to understand.
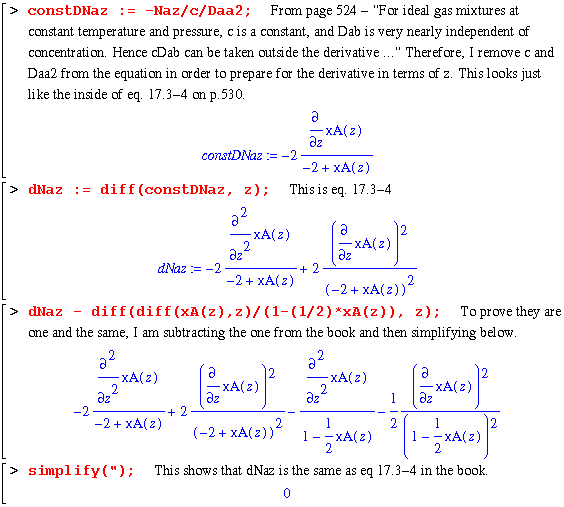
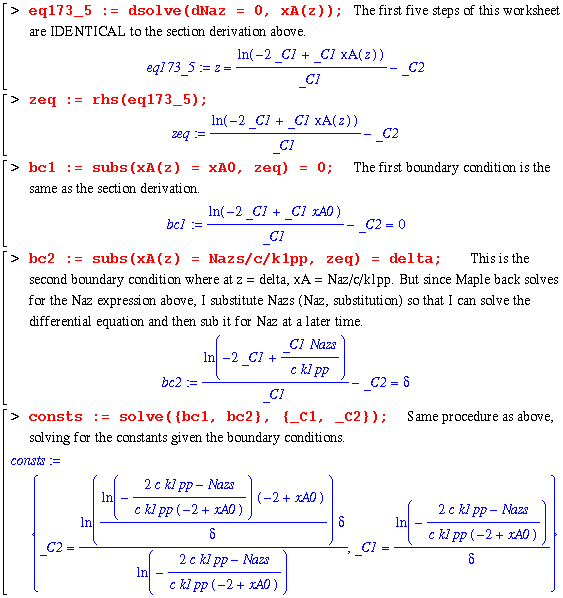
So this puts the worksheet at the step of solving this differential equation for the 2 constant terms by applying the following boundary conditions:
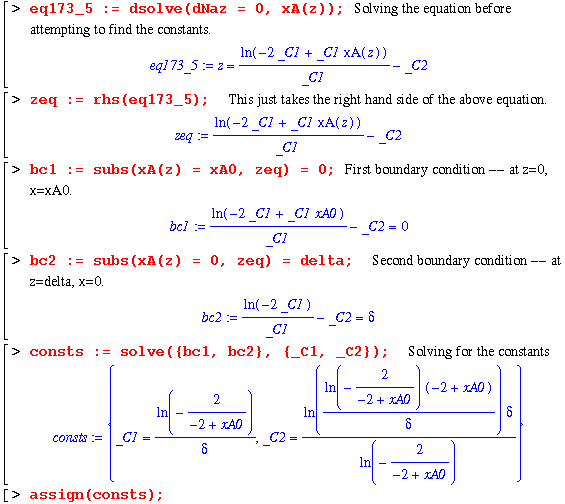
Finally, the constants are determined and a complete expression for xA as a function of z is explicit. In order to check this solution, I will now use my equation for xA in the same manner as eq. 17.3-8 : 1 - (1/2)*xA. Then, comparing this with the book's right hand side of eq. 17.3-8 should yield a zero.
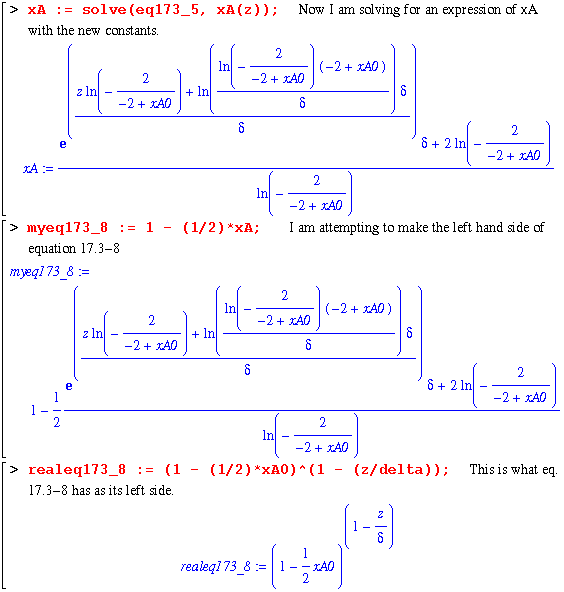
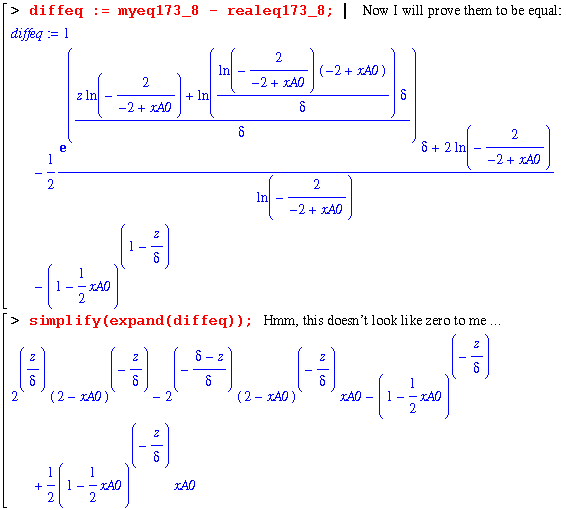
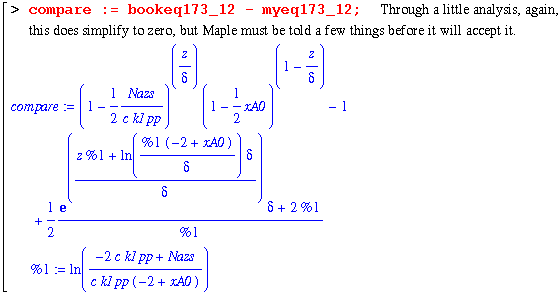
Note that Maple refuses to give a zero! But looking at the following two lines, it becomes obvious that the first expression does simplify to zero.
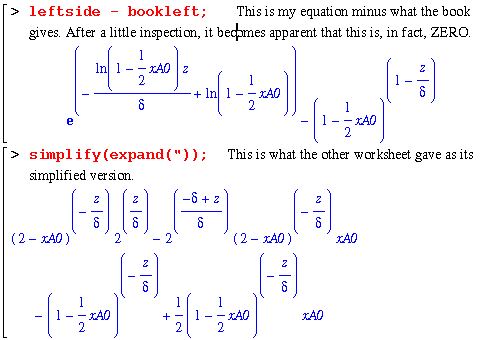
Alas, we are forced to accept that these were equal even though Maple refuses to acknowledge this.
Then, from eq. 17.3-8 to 17.3-9 is a quick derivation:
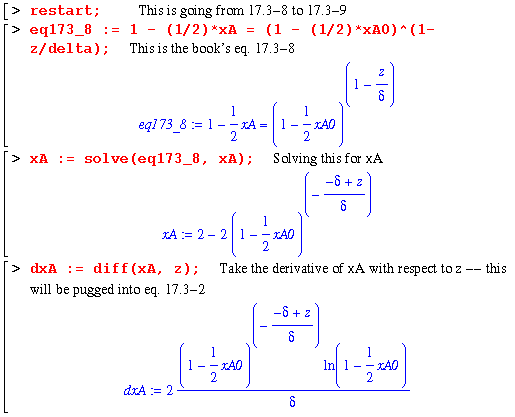
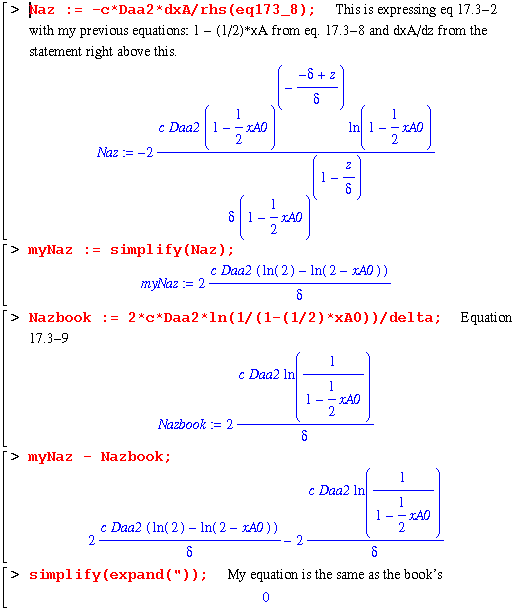
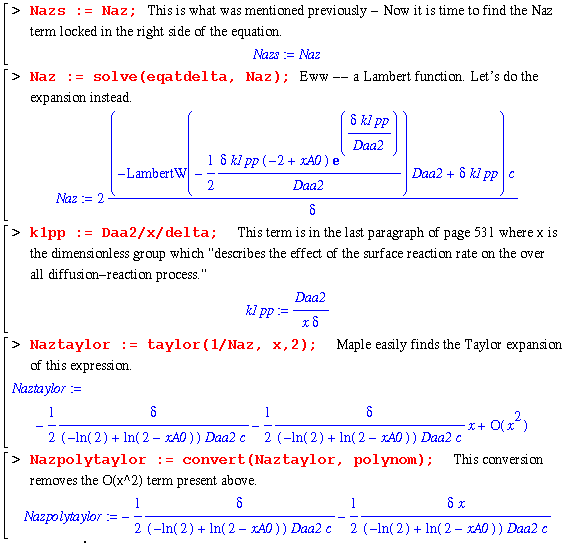
Now begins the example derivation on page 531. This example explores the expressions present when the reaction at the catalyst surface is dependent on the rate, k1'' and the concentration of A. So, we have the same variables and setup as above except that the second boundary condition is now :
So, as above, we set out in the same manner and hope to agree with the book ...
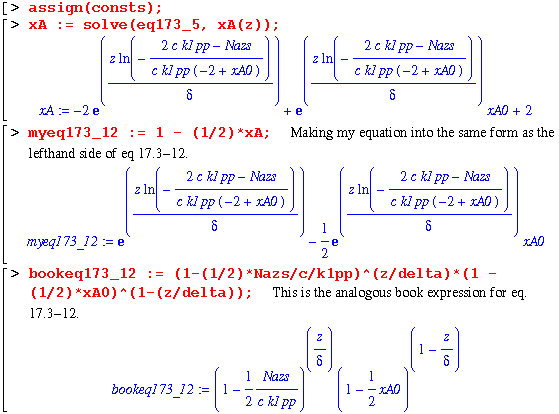
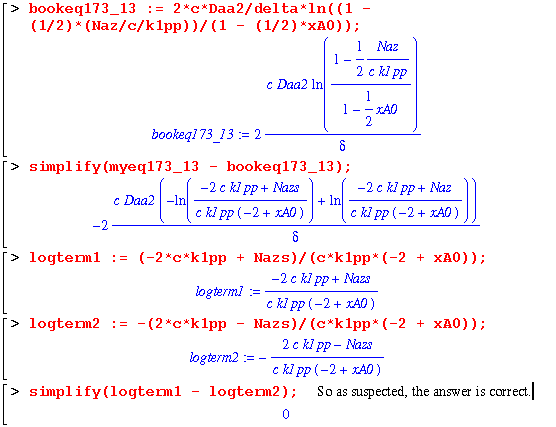
To derive the rest of the equations (eq. 17.3-13 and 17.3-14) in this example, we must approach the problem in a little different way. For equation 13, we start by just solving the Naz equation (eq. 17.3-2) without the fact that the derivative of it with respect to z is equal to zero. This allows us to only worry about one integration and, therefore, one boundary condition.

This is the same as the book, but in a little different form. To further convince ourselves, we can examine the natural log terms to make sure they are the same...
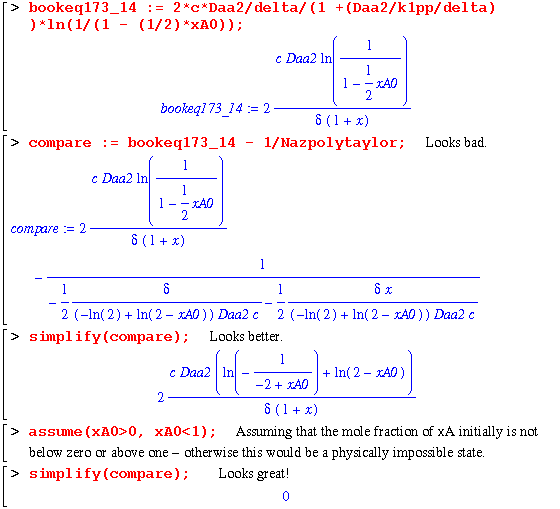
Finally, the last equation requires a good bit of work. The authors note that when the rate constant, k1pp, is large, one can expand the log term into a Taylor series. This is accomplished in the following worksheet:
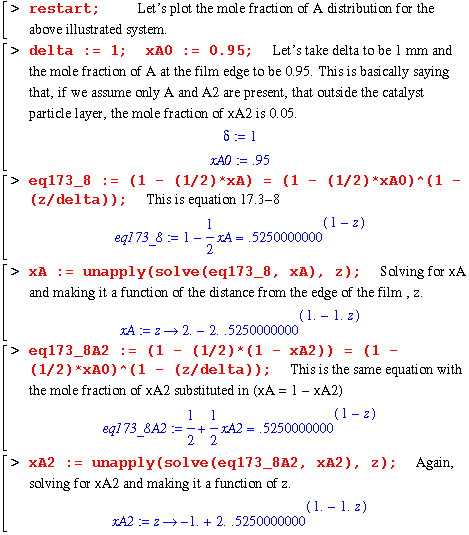
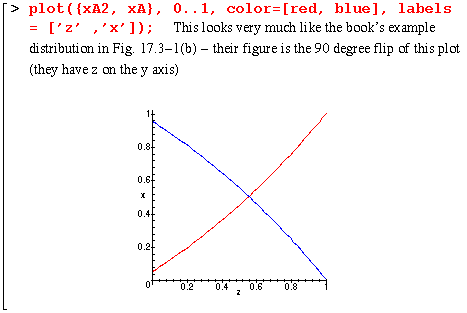
Here is a quick numerical example illustrating the mole fraction distributions of A and A2 within the film. I take xA0 to be .95 at the film's edge and the height of the film, delta, to be 1 mm. I also assume that the reaction at the surface is instantaneous, thereby allowing me to use eq. 17.3-8.