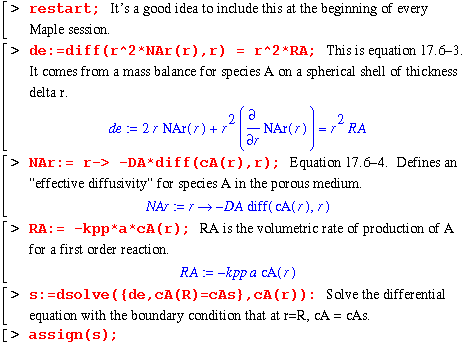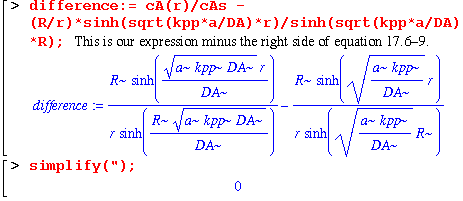DIFFUSION AND CHEMICAL REACTION INSIDE A POROUS CATALYST
Section 17.6; Bird, Stewart and Lightfoot
This section involves derivation of an expression for the concentration and molar flow of component A over a spherical catalyst particle of radius R.
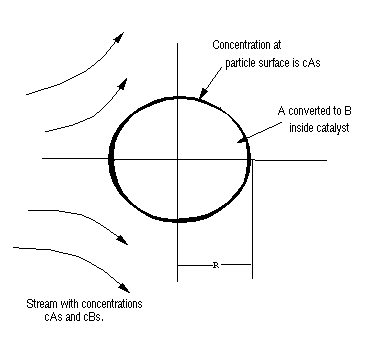
Spherical porous catalyst particle of radius R. At the surface of the catalyst particle, the concentration is cAs moles per unit volume. A diffuses through the tiny passages in the catalyst particle and is converted to B.
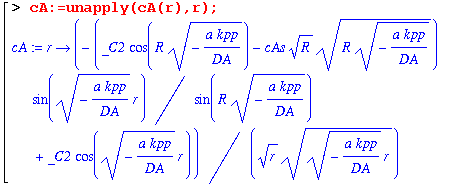
This answer is complicated, so we'll do a few things to try to simplify it.
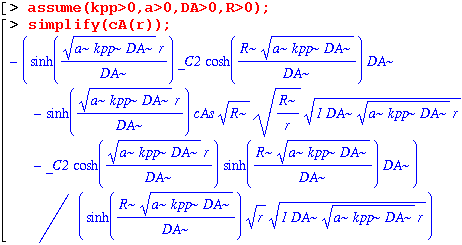
This is still a mess, so we will have to find the constant of integration by taking the limit as r goes to zero.
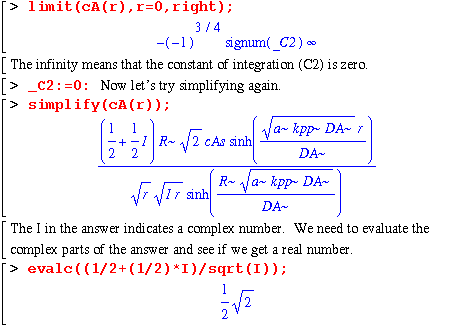
We get a real number, so we know that all of the complex parts cancel. So that I don't have any problems when I try to plug numbers in, I'm going to delete the imaginary parts from cA(r) and multiply by the number above. That should take care of the imaginary problem.
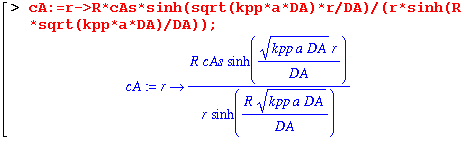
Now we can almost see without calculation that our equation matches that in the book. Let's check just in case. We need to subtract our expression for cA from the one in the book. If they are the same (as they should be), the difference between them should be zero.
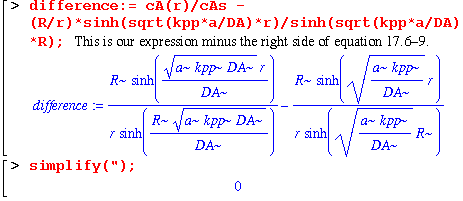
We get zero, so our expression for cA(r)/cAs is the same as the book's. (Equation 17.6-9). Now let's make sure that our expression for WAs, which is the molar flow at the surface r=R, is the same as equation 17.6-11.
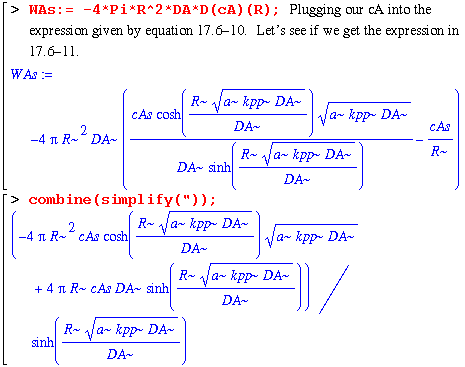
Nope. We'll have to do it the way we did above; by subtracting the book's answer from ours and hoping we get zero.
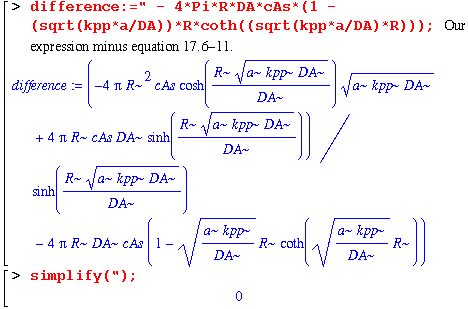
Equation 17.6-11 also agrees with our answer. Now let's do a numerical example.
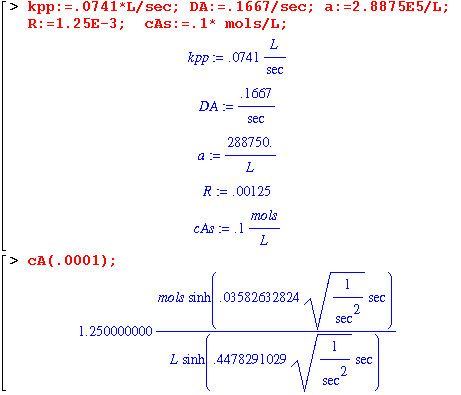
I couldn't get rid of those seconds no matter what I tried. We can see that the units of cA come out correctly in moles per liter, so I'm going to recalculate and not put units on the constants.
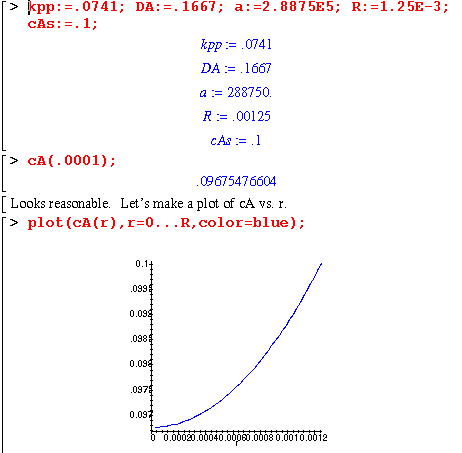
Finally, a representation of cA as r increases.