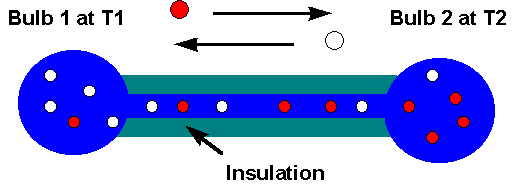
PROBLEM: Two bulbs are joined by a small insulated tube. The bulbs are filled with a mixture of ideal gases A and B, and maintained at constant temperatures T1 and T2. Convection is neglible. Derive the steady-state gas composition for the two bulbs.

SOLUTION: Simplify Eq. 18.4-15 to find the mass flux caused by the temperature gradient in the system.
(1) Take out the first three terms within the brackets of Eq. 18.4-15. These terms correspond to ordinary (concentration dependant), forced and pressure diffusion, none of which apply to this initial situation.
(2) This leaves the thermal diffusion term kT* DEL (ln T) multiplied by constants.
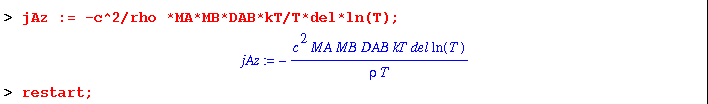
(3) This term simplifies to kT*d(lnT)/dz in one-dimension.
(4) Then kT*d(lnT)/dz = kT/T*dT/dz.
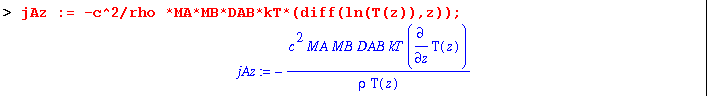
(5) The mass flux now contributes to a concentration gradient, which results in an opposed flux. As defined by Eq. 18.4-15 :
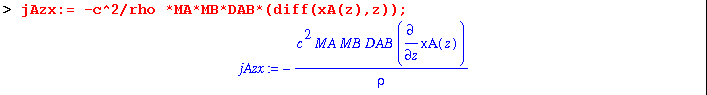
(6) Once at steady-state, there is no net flux. So set the sum of the two fluxes equal to zero:
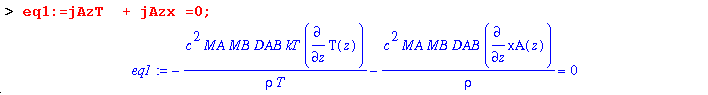
When kT, the thermal diffusion ratio is positive, gas A moves to the cold region. Conversely, when kT is negative, A moves to the hot area. The above equation (eq1) can be simplified to:
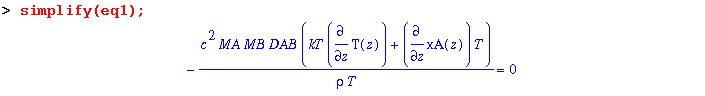
Dividing through by c^2*MA*MB*DAB/(rho*T) yields:
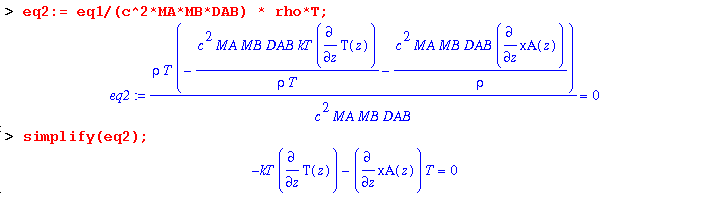
Ignoring the effect of composition on kT and integrating gives: Maple will not integrate and solve eq2; so, by hand, multiply both sides of (18.5-13) by dz. Then integrate the left hand side from xA1 to xA2 and the right hand side from the corresponding temperatures: T1 to T2. Use two assumptions:
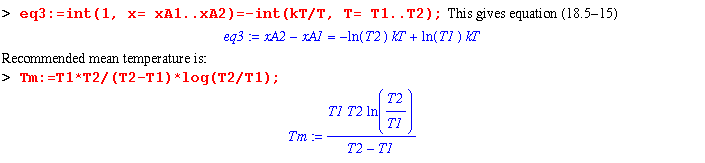
Assume that gas A is nitrogen and gas B is helium. What is the steady-state separation of nitrogen and helium at one atm when T1 = 298 K, T2 = 500 K and the effective average thermal conductivity is 0.0757 (found by "mixkt" with the mole fraction of nitrogen = 0.5)? What should the mean temperature have been for this thermal conductivity?