Questions:
1. What format should the final report be? How much detail about the calculation should we put in it?
3. How do I do 14.2 without knowing how much coal is fed into the furnace?
4. When talking about the combustion of the coal in 14.3, are we expected to factor in the Oxygen within the coal to the combustion equation?
5. What is “the degree of superheat of the wet air” in 14.4 (b)?
6. For 14.4 (c), what state of the air should we use to calculate its feed rate?
7. 14.6 (a)
9. 14.6 (d)
10. 14.7
12. How do I solve 14.9? The unknown temperature of flue gas as it leaves the heat exchanger is needed for the energy balance!
14. What’s the feeding rate of dry coal for 14.14? I can’t find it in the problem description.
15. How do I solve 14.14(b)? It seems to be a pretty complicated system …
16. For 14.14(b), where can I find the heat of combustion for methane?
17. 14.14(c)
19. In 14.18, what’s difference between the 39% efficiency and the efficiency asked by this problem?
20. Can you give us some hint in answering 14.19?
The following steps should generally be followed when
writing your report for those problems on mass/energy balance:
·
Specify the system you do the
mass/energy balance on (by figure or just say it).
·
Show the overall mass/energy balance
equation.
·
Specify the input and
output. For mass balance, list the names of the components and their flow
rates; for energy balance, list the name, flow rate, specific enthalpy and
enthalpy of each component. In the cases when you need to use results (e.g. the
flow rates) calculated in a previous problem, you can just say “The flow rates
are calculated in Problem XXX” and then copy them over.
·
Show the results for each term in the overall mass/energy balance
equation.
·
Show the result for the unknown term in the overall mass/energy
balance equation.
·
Show the method by which you calculate the wanted flow rate,
temperature or whatever that is asked by the question.
·
Show the final answers.
An example is
given below:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- About the flow chart in 14.1, how much information about the equipment’s inlet and outlet should I put in there?
You need to show in the flow chart what is fed into and what is released from the equipment, but you don’t have to specify its detailed compositions. For example, you can draw the furnace like this:
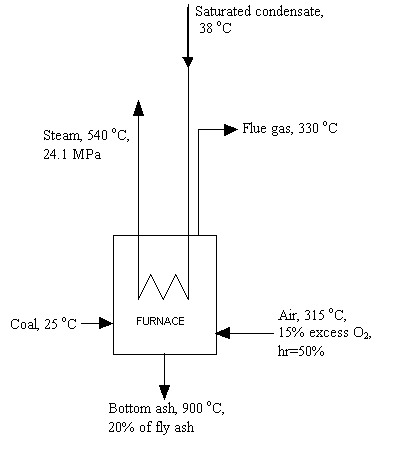
Also, you may want to use
different colors and different line thickness to highlight the main equipment
and the main flow stream.
Right above 14.1, there is a line that says, “Problems 14.2
through 14.10 should be solved using a basis of 100
kg dry coal/min fed to the furnace.” So once
again, read the process description and the problems very carefully.
- When
talking about the combustion of the coal in 14.3, are we expected to factor in the Oxygen within the
coal to the combustion equation?
Yes, you should.
The degree of superheat = The Wet Air’s Temperature – The Wet Air’s dew point = T- Tdew
Use
the state before the air enters the heater exchanger, i.e. its temperature is
25 oC and pressure is 1 atm.
- Do we need to
consider the amount of CaCO3 and CaSO3 dissolved in
the water when doing the mass balance for Problem 14.6(C)?
If yes, what is the solubility for each of them?
Yes,
you should consider the dissolved CaCO3 and CaSO3 for the
mass balance. For the solubility, you can use
CaCO3:
0.002 kg /100 kg water
CaSO3:
0.003 kg /100 kg water
Some students found this problem
particularly confusing and hard to solve. Here is a little help (Of course, you
don’t have to follow the steps below. It will be great if you can find other
ways to solve this problem.)
·
Establish
the following system (the figure) to do mass balance on.
·
Do mass balance on H2O and find out the flow rate of water leaving the scrubber.
·
Do mass balance on CaCO3
and CaSO3, respectively.
·
Find a way to calculate the
combined mass flow rate of CaCO3 and CaSO3×1/2H2O in the slurry
leaving the scrubber. Note
that you don’t have to know the flow rate for each of them. Instead, you only
need to know their combined flow rate.
·
Calculate
the solid-liquid ratio in the slurry leaving the scrubber.
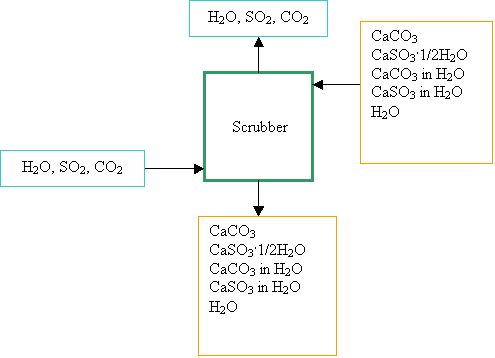
- For 14.6(d), before you embark on a mass balance on
the blending tank for CaCO3, go back to the process description
and look for this sentence: “The fresh ground limestone is fed to the blending tank at a
rate that is 5.2% in excess of that required to react with the SO2 from
the flue gas.”
- For 14.7, the basic energy balance should be (Enthalpy in) – (Enthalpy out)
+ (Heat generation) – (Heat to steam) = 0.
·
Enthalpy
in and Enthalpy out need to be calculated for CO2,
H2O in the air, H2O in the coal, N2, O2,
SO2 and ash (Note that both the states and the flow rates of these
components have changed after the combustion.). You need to define a reference
state in order to calculate enthalpy. To make things easier, define 25C, 1atm
and liquid as the reference state for water and 25C, 1atm for everything else.
Then, use Table B.2 to calculate Cp for each component and with that, calculate
the enthalpy.
·
The
heat generation can be calculated using the HHV value of dry coal.
·
To
calculate the rate of steam generation, you need to find out the specific
enthalpy of the saturated condensate entering the furnace and the super-heated
steam coming out of the furnace. You can find these parameters in the steam
table in the book (use linear interpolation if the exact temperature and
pressure can not be found) or use the code given by Dr. Davis to calculate
them.
- How exactly should I do the linear
interpolation to find the enthalpy of the 24.1Mpa, 540C steam for 14.7? Also, where can I find the enthalpy of
the 38C condensate?
In
Table B.7 (pp. 651), you can find the following specific enthalpies (kJ/kg) of
the superheated steam: (x1, x2 and x are
unknown enthalpies.)
------------------------------------
500C 540C
550
221.2bar | 3210
x1 3370
241 bar
| x
250 bar
| 3166 x2 3337
------------------------------------
The
linear interpolation:
(3370-x1)/(x1-3210)=(550-540)/(540-500)
=> x1=3338 kJ/kg
(3337-x2)/(x2-3166)=(550-540)/(540-500)
=> x2=3302.8 kJ/kg
(x1-x)/(x-x2)=(221.2-241)/(241-250) => x=3313.8 kJ/kg
For 38C
liquid water, its enthalpy (relative to 0C liquid water) can be found in Table
B.5 (pp. 642). It is 159.1
kJ/kg.
Also,
it can be calculated using the specific enthalpy of liquid water in Table B.2
(pp. 637): 75.4/1000*(38-0)=2.8652 kJ/mol = 159.18 kJ/kg
So the enthalpy change from the condensate to the superheated steam = 3313.8-159.18=3154.62 kJ/kg
- How
do I solve 14.9? The unknown
temperature of flue gas as it leaves the heat exchanger is needed for the
energy balance!
The
only way to do this is the trial and error method. You assume a temperature for the flue gas and see if the enthalpy
in and enthalpy out are balanced. If not, you'll have to change the temperature
and try again. The process continues until you find the right answer. I would
suggest you use Excel or even write a small Matlab program to do this. The same
method should be used to solve 14.14 (b). Of course, in that case the unknown is the rate at which methane
is burned.
- For 14.11, do we have to go through all the
calculations for the new feeding rate of coal when the efficiency of the
power plant is 39%?
I think the best way to do this
problem is to first find out the factor by which the feeding rate of coal needs
to be increased and then to multiply all the results you’ve got for 100 kg dry
coal/min feeding rate by that factor.
For
14.14, you should use the required
feeding rate calculated in 14.11.
The 100kg dry coal/min is just for 14.2
through 14.10.
Well, as always, first draw a
figure (like the one below) for the system you are studying. Then do the mass
and energy balances.
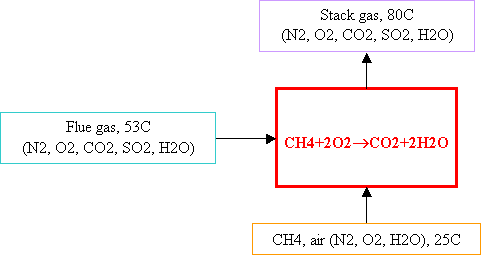
In Table B.1 of the
textbook. Note that what you can find there is combustion heat with H2O(liquid)
as a product while what you need is
combustion heat with H2O(gas) as a product. You should find out how
to convert one to the other.
- When
solving 14.14(c), you need to assume that all
the heat produced by burning the extra amount of coal is transported to
the scrubbed flue gas by the steam. So the energy balance here should be Enthalpy in in
flue gas + Enthalpy transported by steam = Enthalpy out in flue gas.
Note that when more coal is burned in the
furnace, the amount of flue gas will be increased.
- In 14.17, how can I calculate the size (kW and
horsepower) of the pump without knowing the height by which the condensate
needs to be raised?
That’s one of the simplifying
assumptions you need to make when using Bernoulli equation (below) to solve this problem.
DU + gDz + D(u2)/2 + D(pv)
= Qe + We
Find out what are the physical
meanings of these characters and how to simplify this equation by making
reasonable assumptions. Note that the pressure and specific volume of the
condensate and the superheated steam can be found in the Appendix of the book.
For the specific volume of the steam, interpolation method similar to the one
in Q&A #10 should be used. Also note that the flow rate of the
condensate/steam should be the scaled-up number from 14.11, not from 14.7.
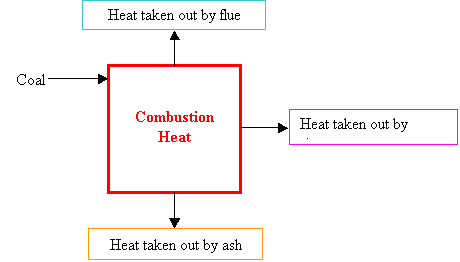
The
39% efficiency is an overall efficiency of the power plant. It means (in 14.11) that “for each unit of heat
released with the combustion of coal, 0.39 unit is converted to electrical
energy”. In the following figure, it can be calculated as 500MWe/(Combustion Heat). The efficiency 14.18 asks for can be expressed as for each unit of
heat transferred to the power generating system by the steam, what percentage
of it is converted to electrical energy. In the
following figure, it can be calculated as 500MWe/(Heat
taken out by steam).
Actually 14.19 itself
gives some information for its answer. It asks you to “include in your
discussion on the impact of the regulation on the use of clean coal”.
Obviously, the power plants can’t use very dirty coals because of the 520 ng SO2/J
HHV criterion. Dirty coal has lower HHV and higher S percentage. As to the 90%
SO2 removal criterion, I think the government just wants the power
plants to remove most of the SO2 in their stack gas even though they are using
clean coals and are producing very small amount of SO2. In a word, these
two criteria are there to prevent power plants from releasing too much SO2
into the atmosphere.