 Home
Home
The hardest thing to learn in life is
which bridge to cross and which to burn.
David Russell
Day 5: Anion Exchange Chromatography
Assignments Due
- Methods for measuring protein concentration (Scopes 2nd ed. pp. 278-283; 3rd ed. pp. 44-50)
- Study Guide (bring a copy of this to lab)
- Results (McMillan: pp. 30-50, 66-71,
81-83, 126-160 (3rd ed.); 45-67, 83-89, 108-109, 167-205 (4th
ed.))
See Research Paper .
Preparation
- Writing the Results
- Anion exchange chromatography set-up
- Protein determination
Overview of Experiment
Run ion exchange columns using a salt gradient to elute adenosine deaminase. Complete all the measurements needed to complete a purification table for your enzyme preparation including activity measurements for all stored samples and the Q-cartridge fractions. Determine protein concentrations for all samples.
Procedures
The chromatography systems have been plumbed for running the
ion exchange cartridge. All components of the station will
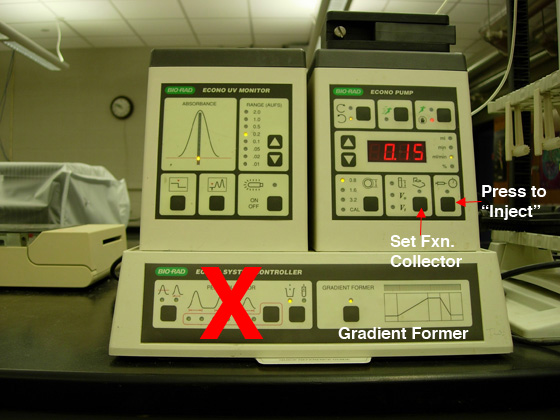
be used except for the "peak collection" option, marked with
the red "X" (see Fig. 1). Each team
will share an instrument. The same conditions should be used
for both native and cloned preparations and the samples will
be run sequentially. Construct a 0.5 M salt buffer (250
ml is sufficient volume): add salt to your anion exchange buffer.
 |
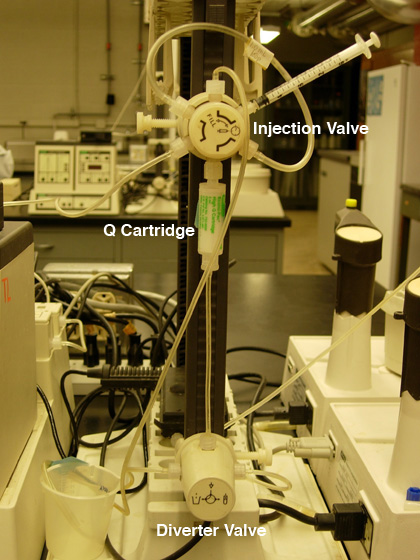 |
Fig. 1. A) Pump settings for anion exchange
chromatography. |
B) Q cartridge hook-up. |
Preparation of Sample
- Wearing gloves, rinse the outside of the dialysis tubing with RO water.
- Recover the residue from the dialysis tubing with a MINIMUM
of anion exchange buffer.
A total final volume of less than
1 ml is recommended. Achieve a more quantitative recovery by
rinsing the tubing with more than one addition of fresh buffer
(e.g., three rinses with 0.3 ml or two rinses with 0.5 ml are
better than one addition of 1 ml).
- Mix the recovered suspension
well to dissolve the residue.
- Centrifuge in a 1.5 ml tube for
5 minutes at maximum speed to pellet any insoluble material.
- Transfer the supernatant to a clean 1.5 ml tube.
The sample is ready for loading onto the Q cartridge.
Caution: Do not shake to cause foaming while attempting
to dissolve the material. Foaming indicates the presence of
denatured protein that will act as a detergent to denature
even more protein.
Q Cartridge Set-Up
NOTE: Use ONE pump station per team and run columns sequentially. Use
ONLY the "slow-running man" when operating the pump today (i.e.,
do NOT ever use the "fast-running man" = purge).
***Set the UV monitor: select 0.05 for RANGE; ZERO the monitor
as on day 3.***
- Install your buffers in the chromatography system (Fig.
2). Buffer "A" is
anion exchange buffer (the loading buffer) and Buffer "B" is
anion exchange buffer with 0.5 M salt (the eluting buffer). NOTE:
the pump tubing diameter is 1.6 mm.
By DEFAULT, the pump pulls through the Buffer A line;
to switch between buffers A and B, press the "Gradient Former" button
while the pump is running.
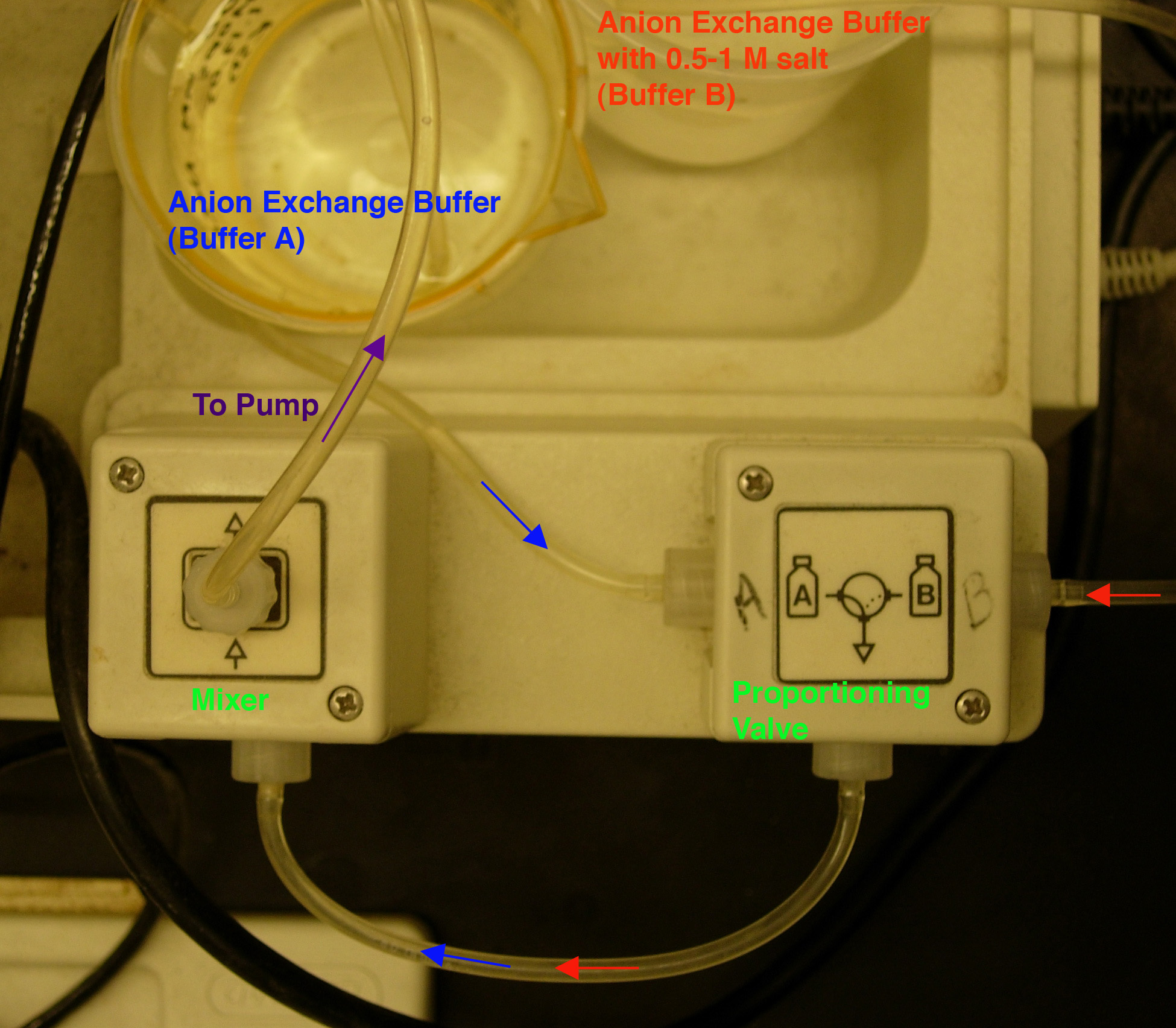
Fig. 2. Buffer set-up.
- Set the flow rate to 6 ml/min.
- Disconnect the top of
the Bio-Rad Econo-Pac Q cartridge (5 ml) from
the system and flush the lines with both buffers (this
step will only take a few seconds).
- Reconnect the Q cartridge and
rinse with Buffer B for 3
minutes then rinse with Buffer A for 3 minutes (flow rate =
6 ml/min). The column is now equilibrated in your starting
buffer. You will need to rinse the cartridge again between
sample loadings.
Remember to return to the desired flow rate before loading the second sample.
- Set the flow rate for your run; Bio-Rad recommends
0.5-3 ml/min for the 5 ml Q cartridges.
- Program a gradient of no more than 30 minutes; you can program
up to FIVE steps (Fig. 3).
 |
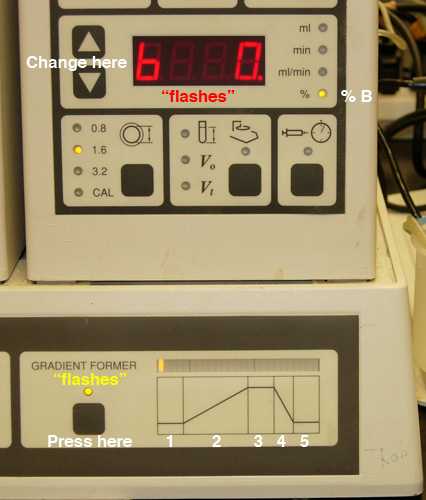 |
Fig.
3. Setting the gradient: A) Selection of "time" for
a given step. |
B) Selection of "% b" (Buffer B) for a given step. |
- Set the fraction
volume ("test tube") using the pump (*you
do not need to set Vo, Vt, or # fractions), zero the UV monitor,
and activate the pump (turn "on" using
the slow running man).
NOTE: Make sure you see a "dashed, red line" on the fraction
collector LED (see Fig. 4).

Fig. 4. Appearance of the fraction collector when it's
controlled by the pump.
- Be certain the injection valve is in the "Fill" position (CCW) then
load your sample into the injection loop (maximum of 1.3 ml)
using the syringe (see Fig. 5A).
DO NOT REMOVE THE SYRINGE FROM THE PORT!!
- Check that the fraction collector is ready and the chart
recorder is on; then move the injection valve to the "Inject" position (CW),
and push the blinking "Inject" button to
start the gradient (see Fig. 5B).
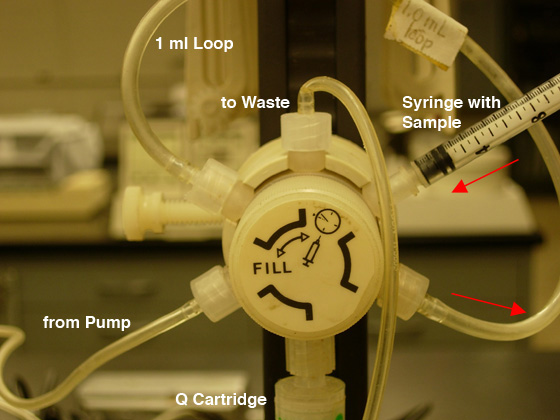 |
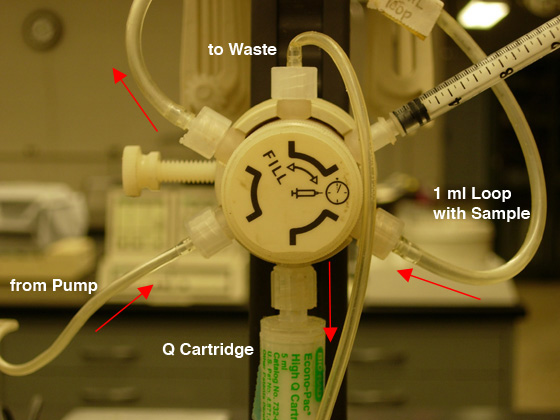 |
Fig.
5. Sample loading: A) FILL the LOOP (CCW position).
|
B) INJECT the COLUMN (CW position). |
Observe that the sample is being rinsed into the cartridge and that the fraction collector is started.
Monitor the progress through the first two or three tubes to be certain of function and alignment of the fraction collector.
- Use the Qualitative Spectrophometric Assay for ADA Activity (SAME as on Day 3) to find the ADA activity in your fractions.
*YOU MUST USE THE SPECTRONIC GENESYS 5 SPEC'S FOR THIS ASSAY.
**ASSAY ALL FRACTIONS!
- Combine the active fractions and obtain an A280
reading for the combined fraction as you did on Day 3;
this value will be used to determine protein concentration. What
buffer do you use to AUTO ZERO the spec?
SAVE YOUR PROTEIN SOLUTION!!
- Use the ADA activity spectrophotometric assay at
265 nm, as used on Day
1, to obtain QUANTITATIVE
assessment of the activity in EACH STEP of the purification
(NOTE: you do NOT need to use a CONTROL enzyme). Thaw the aliquots
from past steps and keep them on ice. Some samples may have
too much activity to measure even using only 1 µl.
Be prepared to dilute a small fraction of the aliquot to add
at least 10 µl to the assay for the activity
measurement; do NOT dilute ALL of the fraction aliquots. On
the other hand, some samples may have very little activity
in just 20 µl; you may add up to 100 µl of
fraction in the assay.
Obtain activity measurements for crude fraction,
P-60 fraction, and Q fraction for BOTH native and cloned sources:
- The measurements should be linear and in the range of
-0.02 to -0.06 ΔA/min
- CE must be diluted--first try 1:10 for NATIVE and 1:100 for CLONED
- Try undiluted P-60 and Q first; dilute only if necessary
- Remember to save the remainder of all
the UNDILUTED samples for gel and kinetic analyses;
DISCARD any diluted samples and leftover adenosine
NOTE: PERFORM THESE ASSAYS WHILE THE Q CARTRIDGES ARE RUNNING
- Add necessary components for stability (e.g., glycerol, DTT,
beta-mercaptoethanol, sucrose, EDTA) to the final purified
enzyme fraction.
NOTE: a 0.5 M EDTA stock solution has been prepared for your use; you do not need to weigh out DTT--just use a "speck" on the end of a small spatula.
- Store final purified enzyme fraction in 5 ml tubes with screw
caps at either 4°C or -20°C.
NOTE: set aside 50µl of your Q fraction for desalting
(see procedure below).
Desalting of Q Fraction for SDS-PAGE
All samples need to be in similar buffer prior to loading
the SDS-PAGE gel (Lab Day 5).
The buffer composition of the Q fraction must be changed; the sample will be desalted using small columns equilibrated in the Tris/glycine/SDS running buffer.
Quick method for changing buffers of samples for SDS gels:
- Obtain an Ultrafree (0.22 µm) device for
each sample to be processed and insert the column into a 1.5
ml centrifuge tube.
- Using a disposable transfer pipet, fill the upper portion
of the Ultrafree device with a slurry of G-25 beads (about
400 µl).
- Pulse spin (hold the "SHORT" key on the microcentrifuge)
for ~15 seconds in the microfuge to remove excess water; discard
the flow-through (waste water).
- Add 100-200 µl of Tris/glycine/SDS running buffer to the gel material and let stand for 1-2 minutes.
- Remove the excess buffer with a 15-20 second pulse spin.
- Carefully pipet 50 µl of your Q fraction on the gel material
and let stand 2-5 minutes at room temperature.
- Recover the sample in a clean 1.5 ml microcentrifuge
tube with
a 10 second pulse spin.
The sample will be in an acceptably desalted state.
- Store the sample at -20°C (*do NOT put "loose" tubes
in the freezer--use a screw-cap bottle).
- Rinse out the beads with RO water and RETURN the Ultrafree
device to the container.
Copyright, Acknowledgements,
and Intended Use
Created by B. Beason (bbeason@rice.edu),
Rice University, 25 May 2010
Updated 5 May 2012


 Home
Home 






